Environmental Research ( IF 7.7 ) Pub Date : 2021-10-02 , DOI: 10.1016/j.envres.2021.112171 R M Akhmadullin 1 , A T Gubaidullin 2 , Kh E Kharlampidi 3 , S R Kurbankulov 3 , T F Nigmatullin 3 , M U Dao 4 , R F Khamidullin 3 , A G Akhmadullina 1 , Y Vasseghian 5 , H Y Hoang 6
|
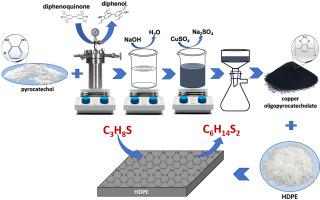
A novel catalyst based on bivalent copper oligopyrocatecholate was first successfully synthesized and dispersed in a polymer matrix for oxidative degradation of mercaptan in aqueous caustic solution. X-ray diffraction analysis has demonstrated that the synthesized catalyst was a crystalline phase with a minimum amorphous component. Mechanism analysis and kinetic experiments were conducted to investigate the kinetic mechanism of the reaction of isopropyl mercaptan oxidation catalyzed by copper oligopyrocatecholate dispersed in a polymer matrix. The influences of temperature, initial concentrations of reactants, and catalytic surface area on the reaction rate were studied to obtain the rate expression of intrinsic kinetics. The research results showed that the subsequent electron-transfer step was the rate-limiting step of the reaction. Additionally, the mercaptan oxidation rate in caustic solution was inversely proportional to the first power of the alkali concentration. The apparent activation energy was approximately 27.71 ± 1.12 kJ/mol. Importantly, this rate law for mercaptan oxidation can be used to design industrial reactors for the light oil sweetening process.
中文翻译:

二价低聚邻苯二酚铜作为一种新型多相催化剂,用于在苛性碱溶液中氧化降解硫醇:合成、表征和动力学研究
首次成功合成了一种基于二价低聚邻苯二酚铜的新型催化剂,并将其分散在聚合物基质中,用于在苛性碱水溶液中氧化降解硫醇。X 射线衍射分析表明,合成的催化剂是具有最少无定形组分的结晶相。通过机理分析和动力学实验研究了分散在聚合物基质中的低聚邻苯二酚铜催化异丙硫醇氧化反应的动力学机理。研究了温度、反应物初始浓度和催化表面积对反应速率的影响,得到了本征动力学的速率表达式。研究结果表明,随后的电子转移步骤是反应的限速步骤。此外,苛性碱溶液中硫醇的氧化速率与碱浓度的一次方成反比。表观活化能约为 27.71 ± 1.12 kJ/mol。重要的是,硫醇氧化的这种速率定律可用于设计轻油脱硫工艺的工业反应器。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号