当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iridium-Catalyzed Regioselective Borylation through C–H Activation and the Origin of Ligand-Dependent Regioselectivity Switching
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-10-01 , DOI: 10.1021/acs.joc.1c02126 Anju Unnikrishnan 1 , Raghavan B Sunoj 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-10-01 , DOI: 10.1021/acs.joc.1c02126 Anju Unnikrishnan 1 , Raghavan B Sunoj 1
Affiliation
|
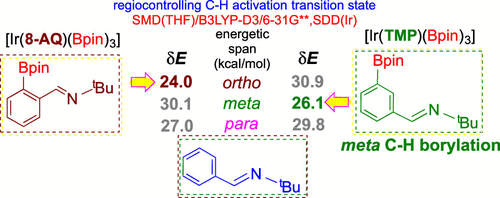
Research efforts in catalytic regioselective borylation using C–H bond activation of arenes have gained considerable recent attention. The ligand-enabled regiocontrol, such as in the borylation of benzaldehyde, the selectivity could be switched from the ortho to meta position, under identical conditions, by just changing the external ligand (L) from 8-aminoquinoline (8-AQ) to tetramethylphenanthroline (TMP). The DFT(B3LYP-D3) computations helped us learn that the energetically preferred catalytic pathway includes the formation of an Ir−π-complex between the active catalyst [Ir(L)(Bpin)3] and benzaldimine, a C–H bond oxidative addition (OA) to form an Ir(V)aryl-hydride intermediate, and a reductive elimination to furnish the borylated benzaldehyde as the final product. The lowest energetic span (δEortho = 26 kcal/mol with 8-AQ) is noted in the ortho borylation pathway, with the OA transition state (TS) as the turnover-determining TS. The change in regiochemical preference to the meta borylation (δEmeta = 26) with TMP is identified. A hemilabile mode of 8-AQ participation is found to exhibit a δEortho of 24 kcal/mol for the ortho borylation, relative to that in the chelate mode (δEortho = 26 kcal/mol). The predicted regioselectivity switching is in good agreement with the earlier experimental observations.
中文翻译:

通过 C-H 激活的铱催化区域选择性硼酸化和配体依赖性区域选择性转换的起源
使用芳烃的 C-H 键活化的催化区域选择性硼化的研究工作最近引起了相当大的关注。配体启用的区域控制,例如在苯甲醛的硼酸化中,在相同条件下,只需将外部配体 ( L ) 从 8-氨基喹啉 ( 8-AQ ) 改为四甲基菲咯啉,就可以将选择性从邻位切换到间位(TMP)。DFT(B3LYP-D3) 计算帮助我们了解到能量优选的催化途径包括在活性催化剂 [Ir( L )(Bpin) 3] 和苯甲醛二亚胺、C-H 键氧化加成 (OA) 以形成 Ir(V) 芳基氢化物中间体,以及还原消除以提供硼化苯甲醛作为最终产物。最低能量跨度(δ E邻位= 26 kcal/mol,8-AQ)在邻硼酸化途径中被注意到,OA 过渡态 (TS) 作为转换决定 TS。确定了对TMP代谢偏硼化 (δ E meta = 26) 的区域化学偏好的变化。的hemilabile模式8-AQ参与发现表现出一个δ Ë邻的24千卡/摩尔的邻硼酸化,相对于螯合模式(δ E邻位= 26 kcal/mol)。预测的区域选择性转换与早期的实验观察结果非常一致。
更新日期:2021-11-05
中文翻译:

通过 C-H 激活的铱催化区域选择性硼酸化和配体依赖性区域选择性转换的起源
使用芳烃的 C-H 键活化的催化区域选择性硼化的研究工作最近引起了相当大的关注。配体启用的区域控制,例如在苯甲醛的硼酸化中,在相同条件下,只需将外部配体 ( L ) 从 8-氨基喹啉 ( 8-AQ ) 改为四甲基菲咯啉,就可以将选择性从邻位切换到间位(TMP)。DFT(B3LYP-D3) 计算帮助我们了解到能量优选的催化途径包括在活性催化剂 [Ir( L )(Bpin) 3] 和苯甲醛二亚胺、C-H 键氧化加成 (OA) 以形成 Ir(V) 芳基氢化物中间体,以及还原消除以提供硼化苯甲醛作为最终产物。最低能量跨度(δ E邻位= 26 kcal/mol,8-AQ)在邻硼酸化途径中被注意到,OA 过渡态 (TS) 作为转换决定 TS。确定了对TMP代谢偏硼化 (δ E meta = 26) 的区域化学偏好的变化。的hemilabile模式8-AQ参与发现表现出一个δ Ë邻的24千卡/摩尔的邻硼酸化,相对于螯合模式(δ E邻位= 26 kcal/mol)。预测的区域选择性转换与早期的实验观察结果非常一致。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号