当前位置:
X-MOL 学术
›
Macromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, Characterization, and Photopolymerization of Polyisobutylene Phenol (Meth)acrylate Macromers
Macromolecules ( IF 5.1 ) Pub Date : 2016-08-15 00:00:00 , DOI: 10.1021/acs.macromol.6b01289 Bin Yang 1 , Corey M. Parada 1 , Robson F. Storey 1
Macromolecules ( IF 5.1 ) Pub Date : 2016-08-15 00:00:00 , DOI: 10.1021/acs.macromol.6b01289 Bin Yang 1 , Corey M. Parada 1 , Robson F. Storey 1
Affiliation
|
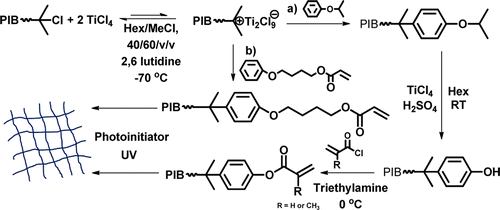
Polyisobutylene (PIB) phenol (meth)acrylates were produced by reacting di- or triphenol-terminated PIB with (meth)acryloyl chloride. 1H NMR, GPC, and MALDI-TOF MS characterization showed that meth(acrylate) end-functionality was 2 and 3, respectively, and that targeted molecular weights and relatively low polydispersities were achieved. Comparative aliphatic PIB triol triacrylate was prepared by end-quenching living polyisobutylene with 4-phenoxy-1-butyl acrylate. A photopolymerization study of PIB diphenol di(meth)acrylates with Mn about 3000 g/mol, PIB triphenol tri(meth)acrylates with Mn about 4000 and 10 000 g/mol, and control aliphatic PIB triol triacrylate with Mn about 10 000 g/mol was conducted. Darocur 1173 and Irgacure 819 and 651 photoinitiators were studied, and FTIR reaction monitoring showed that Darocur 1173 afforded the highest rate of photopolymerization and final conversion, apparently due to its higher solubility in PIB. At Mn ≅ 4000 g/mol, the rate of photopolymerization and conversion of PIB triphenol triacrylate was faster than that of PIB triphenol methacrylate under the same conditions; at Mn ≅ 10 000 g/mol, PIB triphenol triacrylate, PIB triphenol trimethacrylate, and aliphatic PIB triacrylate all showed the same high rate of photopolymerization, which was higher than any rate observed at Mn ≅ 4000 g/mol. Similarly, PIB triphenol tri(meth)acrylate at Mn ≅ 4000 g/mol displayed a higher rate of photopolymerization and double-bond conversion than PIB diphenol di(meth)acrylate at Mn ≅ 3000 g/mol, although they have similar chain end concentrations. This phenomenon was attributed to reduced diffusional mobility at higher Mn, resulting in decreased rate of bimolecular radical termination and autoacceleration. Tg of UV-cured PIB networks decreased as Mn of PIB macromer increased regardless of end-group type, and thermal stability of cured networks remained constant regardless of end-group type. Mechanical properties were characteristic of rubbery networks, but weak, apparently due to low Mn and low PDI of the starting macromers and lack of chain entanglements. Networks from macromers with Mn ≅ 10 000 g/mol gave higher elongations, but lower Young’s moduli, compared to those from macromers with Mn ≅ 4000 g/mol.
中文翻译:

聚异丁烯酚(甲基)丙烯酸酯大分子单体的合成,表征和光聚合
通过使二酚或三酚封端的PIB与(甲基)丙烯酰氯反应,制得聚异丁烯(PIB)酚(甲基)丙烯酸酯。1 H NMR,GPC和MALDI-TOF MS表征表明,甲基丙烯酸酯的末端官能度分别为2和3,并实现了目标分子量和相对较低的多分散性。通过将活性聚异丁烯与丙烯酸4-苯氧基-1-丁酯进行末端淬灭来制备对比例的脂肪族PIB三醇三丙烯酸酯。PIB二酚二(甲基)的光聚合研究丙烯酸酯与中号Ñ约3000克/摩尔,PIB三酚三(甲基)丙烯酸酯与中号Ñ约4000至10000g / mol,并且控制脂族PIB三醇三丙烯酸酯与中号Ñ进行约10000g / mol。对Darocur 1173和Irgacure 819和651光引发剂进行了研究,FTIR反应监测表明Darocur 1173提供了最高的光聚合速率和最终转化率,这显然是由于Darocur 1173在PIB中的溶解度更高。在中号Ñ ≅4000克/摩尔,光聚合反应的速率和PIB的转化三酚三丙烯酸酯比在相同条件下PIB三酚甲基丙烯酸酯的速度更快; 在中号Ñ ≅10 000g / mol的,PIB三酚三丙烯酸酯,三苯酚PIB三甲基,和脂族三丙烯酸PIB所有显示光聚合反应的相同高速率,这比在观察到的任何速率更高中号Ñ ≅4000克/摩尔。同样,PIB三酚三(甲基)丙烯酸酯中号Ñ ≅4000克/摩尔在显示的光聚合和双键转换比PIB二酚二(甲基)丙烯酸酯的较高速率中号Ñ ≅3000克/摩尔,尽管它们具有类似的链端浓度。这种现象归因于在较高的M n下扩散迁移率降低,从而导致双分子自由基终止和自加速速率降低。紫外线固化的PIB网络的T g随PIB大分子单体的M n的增加而降低,而与端基类型无关,而固化的网络的热稳定性无论端基类型如何均保持恒定。力学性能是橡胶网络的特征,但是很弱,显然是由于低Mn和起始大分子单体的PDI较低,并且没有链缠结。从与大分子单体网络中号Ñ ≅10 000g / mol的,得到更高的伸长率,但降低的杨氏模量,比起那些从与大分子单体中号Ñ ≅4000克/摩尔。
更新日期:2016-08-15
中文翻译:

聚异丁烯酚(甲基)丙烯酸酯大分子单体的合成,表征和光聚合
通过使二酚或三酚封端的PIB与(甲基)丙烯酰氯反应,制得聚异丁烯(PIB)酚(甲基)丙烯酸酯。1 H NMR,GPC和MALDI-TOF MS表征表明,甲基丙烯酸酯的末端官能度分别为2和3,并实现了目标分子量和相对较低的多分散性。通过将活性聚异丁烯与丙烯酸4-苯氧基-1-丁酯进行末端淬灭来制备对比例的脂肪族PIB三醇三丙烯酸酯。PIB二酚二(甲基)的光聚合研究丙烯酸酯与中号Ñ约3000克/摩尔,PIB三酚三(甲基)丙烯酸酯与中号Ñ约4000至10000g / mol,并且控制脂族PIB三醇三丙烯酸酯与中号Ñ进行约10000g / mol。对Darocur 1173和Irgacure 819和651光引发剂进行了研究,FTIR反应监测表明Darocur 1173提供了最高的光聚合速率和最终转化率,这显然是由于Darocur 1173在PIB中的溶解度更高。在中号Ñ ≅4000克/摩尔,光聚合反应的速率和PIB的转化三酚三丙烯酸酯比在相同条件下PIB三酚甲基丙烯酸酯的速度更快; 在中号Ñ ≅10 000g / mol的,PIB三酚三丙烯酸酯,三苯酚PIB三甲基,和脂族三丙烯酸PIB所有显示光聚合反应的相同高速率,这比在观察到的任何速率更高中号Ñ ≅4000克/摩尔。同样,PIB三酚三(甲基)丙烯酸酯中号Ñ ≅4000克/摩尔在显示的光聚合和双键转换比PIB二酚二(甲基)丙烯酸酯的较高速率中号Ñ ≅3000克/摩尔,尽管它们具有类似的链端浓度。这种现象归因于在较高的M n下扩散迁移率降低,从而导致双分子自由基终止和自加速速率降低。紫外线固化的PIB网络的T g随PIB大分子单体的M n的增加而降低,而与端基类型无关,而固化的网络的热稳定性无论端基类型如何均保持恒定。力学性能是橡胶网络的特征,但是很弱,显然是由于低Mn和起始大分子单体的PDI较低,并且没有链缠结。从与大分子单体网络中号Ñ ≅10 000g / mol的,得到更高的伸长率,但降低的杨氏模量,比起那些从与大分子单体中号Ñ ≅4000克/摩尔。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号