Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cosolutes Modify Alkaline Phosphatase Catalysis through Osmotic Stress and Crowding Mechanisms
ACS Omega ( IF 3.7 ) Pub Date : 2021-09-30 , DOI: 10.1021/acsomega.1c03243 Oksana A Yavorska 1 , Lukas Syriste 2 , Chantal M du Plessis 1 , Maryam Yaqoob 1 , Kyle Loogman 1 , Michael Cordara 1 , John K Chik 1
ACS Omega ( IF 3.7 ) Pub Date : 2021-09-30 , DOI: 10.1021/acsomega.1c03243 Oksana A Yavorska 1 , Lukas Syriste 2 , Chantal M du Plessis 1 , Maryam Yaqoob 1 , Kyle Loogman 1 , Michael Cordara 1 , John K Chik 1
Affiliation
|
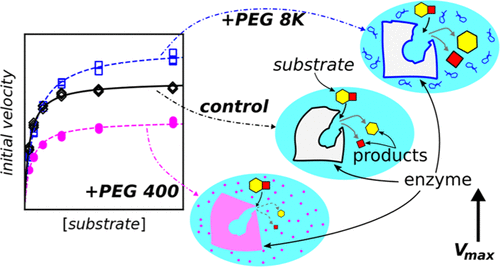
Examining the effects of different cosolutes on in vitro enzyme kinetics yielded glimpses into their potential behavior when functioning in their natural, complex, in vivo milieu. Viewing cosolute in vitro influences on a model enzyme, calf intestinal alkaline phosphatase, as a combination of competitive and uncompetitive behaviors provided quantitative insights into their effects on catalysis. Observed decreases in the apparent specificity constant, Kasp, caused by the presence of polyethylene glycols or betaine in the reaction solution, indicated interference with enzyme–substrate complex formation. This competitive inhibition appeared to be driven by osmotic stress. Dextran 6 K and sucrose strongly impeded the subsequent conversion of the bound substrate into a free product, which was marked by sharp reductions in Vmax, uncompetitive inhibition. For the same step, smaller noncarbohydrate cosolutes, triethylene glycol, polyethylene glycol 400, and betaine, also behaved as uncompetitive inhibitors but to a lesser extent. However, polyethylene glycol 8000 and 20,000 were uncompetitive activators, increasing Vmax. Polyethylene glycol of molecular weight 1000 displayed intermediate effects between these two groups of noncarbohydrate cosolutes. These results suggested that crowding has a strong influence on free product formation. The combination of competitive and uncompetitive effects and mixed behaviors, caused by the cosolutes on calf intestinal alkaline phosphatase kinetics, was consistent with the trends seen in similar enzyme–cosolute studies. It is proposed that the double-displacement mechanism of alkaline phosphatases, shared by many other enzymes, could be the root of this general observation.
中文翻译:

Cosolutes 通过渗透压力和拥挤机制修改碱性磷酸酶催化
检查不同 cosolutes 对体外酶动力学的影响,可以瞥见它们在自然、复杂的体内环境中发挥作用时的潜在行为。观察 cosolute对模型酶、小牛肠碱性磷酸酶的体外影响,作为竞争和非竞争行为的组合,提供了对它们对催化作用的影响的定量见解。观察到表观特异性常数K asp 的降低,由反应溶液中聚乙二醇或甜菜碱的存在引起,表明干扰酶-底物复合物的形成。这种竞争性抑制似乎是由渗透压力驱动的。葡聚糖 6 K 和蔗糖强烈阻碍了结合底物向游离产物的后续转化,其特点是V max急剧下降,非竞争性抑制。对于同一步骤,较小的非碳水化合物共溶物、三甘醇、聚乙二醇 400 和甜菜碱也可作为非竞争性抑制剂,但程度较轻。然而,聚乙二醇8000和20000分别为缺乏竞争力活化剂,增加V最大. 分子量为 1000 的聚乙二醇显示出这两组非碳水化合物共溶质之间的中间效果。这些结果表明拥挤对游离产物的形成有很大的影响。由 cosolutes 对小牛肠道碱性磷酸酶动力学引起的竞争性和非竞争性影响以及混合行为的组合,与类似酶 - cosolute 研究中观察到的趋势一致。有人提出,许多其他酶所共有的碱性磷酸酶的双置换机制可能是这一普遍观察的根源。
更新日期:2021-10-12
中文翻译:

Cosolutes 通过渗透压力和拥挤机制修改碱性磷酸酶催化
检查不同 cosolutes 对体外酶动力学的影响,可以瞥见它们在自然、复杂的体内环境中发挥作用时的潜在行为。观察 cosolute对模型酶、小牛肠碱性磷酸酶的体外影响,作为竞争和非竞争行为的组合,提供了对它们对催化作用的影响的定量见解。观察到表观特异性常数K asp 的降低,由反应溶液中聚乙二醇或甜菜碱的存在引起,表明干扰酶-底物复合物的形成。这种竞争性抑制似乎是由渗透压力驱动的。葡聚糖 6 K 和蔗糖强烈阻碍了结合底物向游离产物的后续转化,其特点是V max急剧下降,非竞争性抑制。对于同一步骤,较小的非碳水化合物共溶物、三甘醇、聚乙二醇 400 和甜菜碱也可作为非竞争性抑制剂,但程度较轻。然而,聚乙二醇8000和20000分别为缺乏竞争力活化剂,增加V最大. 分子量为 1000 的聚乙二醇显示出这两组非碳水化合物共溶质之间的中间效果。这些结果表明拥挤对游离产物的形成有很大的影响。由 cosolutes 对小牛肠道碱性磷酸酶动力学引起的竞争性和非竞争性影响以及混合行为的组合,与类似酶 - cosolute 研究中观察到的趋势一致。有人提出,许多其他酶所共有的碱性磷酸酶的双置换机制可能是这一普遍观察的根源。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号