当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Practical Kilogram Synthesis of (S)-1-Benzyl-4-Bromo-3-Methyl-1,2,3,6-Tetrahydropyridine
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2021-10-01 , DOI: 10.1021/acs.oprd.1c00212 Vincent M. Lombardo 1 , Louise Bernier 2 , Ming Z. Chen 1 , William Farrell 2 , Andrew Flick 1 , Philippe Nuhant 1 , Neal W. Sach 2 , Yong Tao 3 , John I. Trujillo 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2021-10-01 , DOI: 10.1021/acs.oprd.1c00212 Vincent M. Lombardo 1 , Louise Bernier 2 , Ming Z. Chen 1 , William Farrell 2 , Andrew Flick 1 , Philippe Nuhant 1 , Neal W. Sach 2 , Yong Tao 3 , John I. Trujillo 1
Affiliation
|
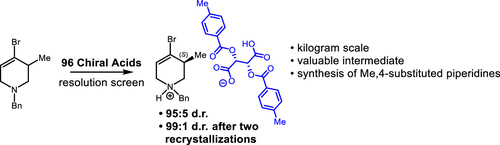
Despite chiral piperidines being a commonly found motif in approved drugs and drug candidates, the synthesis of these compounds occasionally suffers from challenges of scalability and stereochemical control. As part of a medicinal chemistry program, we sought access to a series of stereochemically pure 3,4-disubstituted piperidines. A classical ammonium salt resolution was investigated to furnish chiral (S)-1-benzyl-4-bromo-3-methyl-1,2,3,6-tetrahydropyridine as a valuable chiral precursor to C-3,4 di-substituted piperidines, which may have broader utility for other pharmaceutical intermediates. Specifically, in this work, in support of scale-up efforts for toxicology studies, we describe a practical synthesis to access kilogram quantities of enantiopure (S)-1-benzyl-4-bromo-3-methyl-1,2,3,6-tetrahydropyridine, utilizing a Shapiro reaction, followed by a classical salt resolution with an inexpensive chiral acid in high yield and enantioselectivity.
中文翻译:

(S)-1-Benzyl-4-Bromo-3-Methyl-1,2,3,6-Tetrahydropyridine 的实用千克合成
尽管手性哌啶是已批准药物和候选药物中常见的基序,但这些化合物的合成偶尔会遇到可扩展性和立体化学控制的挑战。作为药物化学计划的一部分,我们寻求获得一系列立体化学纯的 3,4-二取代哌啶。研究了经典的铵盐拆分,以提供手性 ( S )-1-benzyl-4-bromo-3-methyl-1,2,3,6-四氢吡啶作为 C-3,4 二取代哌啶的有价值的手性前体,这可能对其他医药中间体具有更广泛的用途。具体而言,在这项工作中,为了支持毒理学研究的扩大工作,我们描述了一种实用的合成方法,以获取千克数量的对映体纯(S)-1-苄基-4-溴-3-甲基-1,2,3,6-四氢吡啶,利用夏皮罗反应,然后用廉价的手性酸以高产率和对映选择性进行经典的盐拆分。
更新日期:2021-10-15
中文翻译:

(S)-1-Benzyl-4-Bromo-3-Methyl-1,2,3,6-Tetrahydropyridine 的实用千克合成
尽管手性哌啶是已批准药物和候选药物中常见的基序,但这些化合物的合成偶尔会遇到可扩展性和立体化学控制的挑战。作为药物化学计划的一部分,我们寻求获得一系列立体化学纯的 3,4-二取代哌啶。研究了经典的铵盐拆分,以提供手性 ( S )-1-benzyl-4-bromo-3-methyl-1,2,3,6-四氢吡啶作为 C-3,4 二取代哌啶的有价值的手性前体,这可能对其他医药中间体具有更广泛的用途。具体而言,在这项工作中,为了支持毒理学研究的扩大工作,我们描述了一种实用的合成方法,以获取千克数量的对映体纯(S)-1-苄基-4-溴-3-甲基-1,2,3,6-四氢吡啶,利用夏皮罗反应,然后用廉价的手性酸以高产率和对映选择性进行经典的盐拆分。






























 京公网安备 11010802027423号
京公网安备 11010802027423号