当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Deaggregation of Zinc Dihydride by Lewis Acids Including Carbon Dioxide in the Presence of Nitrogen Donors
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2021-09-30 , DOI: 10.1021/acs.inorgchem.1c02207
Florian Ritter 1 , Louis J Morris 1 , Karl N McCabe 2 , Thomas P Spaniol 1 , Laurent Maron 2 , Jun Okuda 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2021-09-30 , DOI: 10.1021/acs.inorgchem.1c02207
Florian Ritter 1 , Louis J Morris 1 , Karl N McCabe 2 , Thomas P Spaniol 1 , Laurent Maron 2 , Jun Okuda 1
Affiliation
|
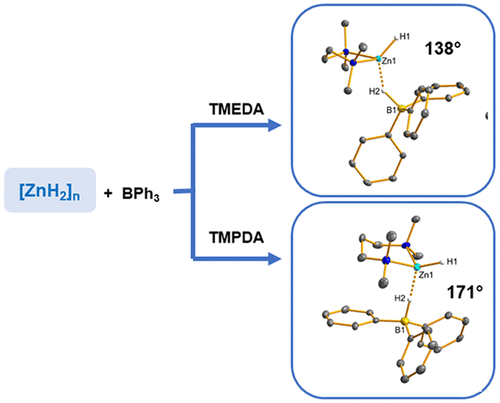
Thermally sensitive polymeric zinc dihydride [ZnH2]n can conveniently be prepared by the reaction of ZnEt2 with [AlH3(NEt3)]. When reacted with CO2 (1 bar) in the presence of chelating N-donor ligands Ln = N,N,N′,N′-tetramethylethylenediamine (TMEDA), N,N,N′,N′-tetramethyl-1,3-propanediamine (TMPDA), N,N,N′,N″,N′′-pentamethyldiethylenetriamine (PMDTA), and 1,4,7,10-tetramethyl-1,4,7,10-tetraazacyclododecane (Me4TACD), insertion into the Zn–H bond readily occurred. Depending on the denticity n, formates [(Ln)Zn(OCHO)2] were isolated and structurally characterized, either as a molecule (Ln = TMEDA, TMPDA, PMDTA) or a charge-separated ion pair [(Ln)Zn(OCHO)][OCHO] (Ln = Me4TACD). The reaction of [ZnH2]n with the mild Lewis acid BPh3 in the presence of chelating N-donor ligands Ln gave a series of hydridotriphenylborates, either as a contact ion pair [(L2)Zn(H)(HBPh3)] (L2 = TMEDA, TMPDA) or a separated ion pair [(Ln)Zn(H)][HBPh3] (Ln = PMDTA, Me4TACD). In the crystal, the contact ion pair [(TMEDA)Zn(H)(HBPh3)] showed a bent Zn–H–B bridge indicative of a delocalized Zn–H–B interaction. In contrast, a linear Zn–H–B bridge for [(TMPDA)Zn(H)(HBPh3)] was observed, suggesting a contact ion pair. In THF solution, both complexes show an exchange with free BPh3 as well as [HBPh3]−. DFT calculations suggest the presence of [HBPh3]− anion with a highly polarized B–H bond that interacts with the Lewis acidic zinc hydride cation [(L2)Zn(H)]+. The hydridotriphenylborates [(Ln)Zn(H)(HBPh3)] underwent CO2 insertion to give (formato)zinc (formoxy)triphenylborate complexes [(Ln)Zn(OCHO)][(OCHO)BPh3] (Ln = TMPDA, PMDTA, Me4TACD). For Ln = TMEDA, a dinuclear complex [(Ln)2Zn2(μ-OCHO)3][(OCHO)BPh3] was isolated. Hydridotriphenylborates [(Ln)Zn(H)(HBPh3)] catalyzed the hydrosilylation of CO2 (1 bar) by nBuMe2SiH in THF at 70 °C to give formoxysilane and (methoxy)silane.
中文翻译:

在氮供体存在下,包括二氧化碳的路易斯酸对二氢化锌的解聚
热敏聚合二氢化锌[ZnH 2 ] n可以方便地通过ZnEt 2与[AlH 3 (NEt 3 )]反应制备。当在螯合 N-供体配体 L n = N , N , N ', N '-四甲基乙二胺(TMEDA), N , N , N ', N '-四甲基-1存在下与 CO 2 (1 bar)反应时, 3-丙二胺 (TMPDA)、N,N,N',N",N'' -五甲基二亚乙基三胺 (PMDTA) 和 1,4,7,10-四甲基-1,4,7,10-四氮杂环十二烷 (Me 4TACD),很容易插入到 Zn-H 键中。根据齿数n,甲酸盐 [(L n )Zn(OCHO) 2 ] 被分离并在结构上进行表征,无论是作为分子 (L n = TMEDA、TMPDA、PMDTA) 还是电荷分离的离子对 [(L n ) Zn(OCHO)][OCHO] (L n = Me 4 TACD)。[ZnH 2 ] n与温和的路易斯酸 BPh 3在螯合 N 供体配体 L n的存在下反应得到一系列氢化三苯基硼酸盐,作为接触离子对 [(L 2 )Zn(H)(HBPh 3 )] (L 2= TMEDA, TMPDA) 或分离的离子对 [(L n )Zn(H)][HBPh 3 ] (L n = PMDTA, Me 4 TACD)。在晶体中,接触离子对 [(TMEDA)Zn(H)(HBPh 3 )] 显示出弯曲的 Zn-H-B 桥,表明离域 Zn-H-B 相互作用。相比之下,观察到[(TMPDA)Zn(H)(HBPh 3 )]的线性 Zn-H-B 桥,表明存在接触离子对。在 THF 溶液中,两种配合物都显示出与游离 BPh 3以及 [HBPh 3 ] -的交换。DFT 计算表明 [HBPh 3 ] -阴离子的存在具有高度极化的 B-H 键,与路易斯酸性氢化锌阳离子相互作用 [(L2 )Zn(H)] +。氢化三苯基硼酸盐 [(L n )Zn(H)(HBPh 3 )] 经历 CO 2插入,得到 (甲氧基) 锌 (甲氧基) 三苯基硼酸盐配合物 [(L n )Zn(OCHO)][(OCHO)BPh 3 ] (L n = TMPDA、PMDTA、Me 4 TACD)。对于 L n = TMEDA,分离出双核复合物 [(L n ) 2 Zn 2 (μ-OCHO) 3 ][(OCHO)BPh 3 ]。氢化三苯基硼酸盐 [(L n )Zn(H)(HBPh 3 )]通过n催化 CO 2 (1 bar)的氢化硅烷化BuMe 2 SiH 在 THF 中在 70 °C 下得到甲氧基硅烷和(甲氧基)硅烷。
更新日期:2021-10-18
中文翻译:

在氮供体存在下,包括二氧化碳的路易斯酸对二氢化锌的解聚
热敏聚合二氢化锌[ZnH 2 ] n可以方便地通过ZnEt 2与[AlH 3 (NEt 3 )]反应制备。当在螯合 N-供体配体 L n = N , N , N ', N '-四甲基乙二胺(TMEDA), N , N , N ', N '-四甲基-1存在下与 CO 2 (1 bar)反应时, 3-丙二胺 (TMPDA)、N,N,N',N",N'' -五甲基二亚乙基三胺 (PMDTA) 和 1,4,7,10-四甲基-1,4,7,10-四氮杂环十二烷 (Me 4TACD),很容易插入到 Zn-H 键中。根据齿数n,甲酸盐 [(L n )Zn(OCHO) 2 ] 被分离并在结构上进行表征,无论是作为分子 (L n = TMEDA、TMPDA、PMDTA) 还是电荷分离的离子对 [(L n ) Zn(OCHO)][OCHO] (L n = Me 4 TACD)。[ZnH 2 ] n与温和的路易斯酸 BPh 3在螯合 N 供体配体 L n的存在下反应得到一系列氢化三苯基硼酸盐,作为接触离子对 [(L 2 )Zn(H)(HBPh 3 )] (L 2= TMEDA, TMPDA) 或分离的离子对 [(L n )Zn(H)][HBPh 3 ] (L n = PMDTA, Me 4 TACD)。在晶体中,接触离子对 [(TMEDA)Zn(H)(HBPh 3 )] 显示出弯曲的 Zn-H-B 桥,表明离域 Zn-H-B 相互作用。相比之下,观察到[(TMPDA)Zn(H)(HBPh 3 )]的线性 Zn-H-B 桥,表明存在接触离子对。在 THF 溶液中,两种配合物都显示出与游离 BPh 3以及 [HBPh 3 ] -的交换。DFT 计算表明 [HBPh 3 ] -阴离子的存在具有高度极化的 B-H 键,与路易斯酸性氢化锌阳离子相互作用 [(L2 )Zn(H)] +。氢化三苯基硼酸盐 [(L n )Zn(H)(HBPh 3 )] 经历 CO 2插入,得到 (甲氧基) 锌 (甲氧基) 三苯基硼酸盐配合物 [(L n )Zn(OCHO)][(OCHO)BPh 3 ] (L n = TMPDA、PMDTA、Me 4 TACD)。对于 L n = TMEDA,分离出双核复合物 [(L n ) 2 Zn 2 (μ-OCHO) 3 ][(OCHO)BPh 3 ]。氢化三苯基硼酸盐 [(L n )Zn(H)(HBPh 3 )]通过n催化 CO 2 (1 bar)的氢化硅烷化BuMe 2 SiH 在 THF 中在 70 °C 下得到甲氧基硅烷和(甲氧基)硅烷。



































 京公网安备 11010802027423号
京公网安备 11010802027423号