背景与目标
男性和女性对非酒精性脂肪性肝病 (NAFLD) 易感性不同的分子机制知之甚少。TTC39B基因座编码一种支架蛋白,与妇科疾病相关,其缺失可保护小鼠免受饮食诱导的脂肪性肝炎。本研究旨在阐明将 TTC39B (T39) 与脂肪生成基因表达联系起来的分子机制,并探索性别特异性效应。
方法
HEK293A 细胞中的共表达验证了蛋白质-蛋白质相互作用算法预测的新型 T39/pRb 相互作用。在患有饮食 NAFLD 和 pRb 或其下游效应子 E2F1 遗传缺陷的小鼠以及原代人肝细胞中,使用反义寡核苷酸 (ASO) 敲低了 T39。
结果
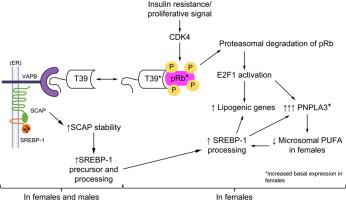
T39 通过其 C 端 TPR 结构域与 pRb 相互作用并促进其蛋白酶体降解。在雌性小鼠中,T39 缺乏会以 pRb 和 E2F1 依赖的方式降低脂肪生成基因的 mRNA,尤其是Pnpla3。相反,在雄性小鼠中,T39 缺乏导致脂肪生成基因表达的减少要小得多,这与 pRb/E2F1 无关。T39 还通过 N 端 FFAT 基序与 VAPB 相互作用,并稳定 VAPB 与 SCAP 的相互作用。卵巢切除术消除了 T39 敲低对肝 pRb/E2F1/ Pnpla3轴的影响。在两种性别中,T39 敲低都会独立于 pRb 减少 SCAP。在原代人肝细胞中,T39 敲低会降低女性而不是男性中PNPLA3和其他脂肪生成基因的表达。
结论
我们发现了肝脏脂肪生成基因调控中保守的两性二态性,T39 的作用通过女性的 pRb/E2F1 和两性的 VAPB/SCAP 介导。T39 抑制可能是下调 PNPLA3 和治疗女性 NAFLD 的新策略。
总结
在女性中,蛋白质 TTC39B 会降解肝脏中的一种肿瘤抑制因子,以促进新脂肪的合成和非酒精性脂肪肝疾病的主要遗传风险因素的表达。TTC39B 是非酒精性脂肪肝的潜在治疗靶点,尤其是女性。
 "点击查看英文标题和摘要"
"点击查看英文标题和摘要"
TTC39B destabilizes retinoblastoma protein promoting hepatic lipogenesis in a sex-specific fashion
Background & Aims
Molecular mechanisms underlying the different susceptibility of men and women to non-alcoholic fatty liver disease (NAFLD) are poorly understood. The TTC39B locus encodes a scaffolding protein, associates with gynecological disorders and its deletion protects mice from diet-induced steatohepatitis. This study aimed to elucidate the molecular mechanisms linking TTC39B (T39) to the expression of lipogenic genes and to explore sex-specific effects.
Methods
Co-expression in HEK293A cells validated the novel T39/pRb interaction predicted by a protein-protein interaction algorithm. T39 was knocked down using an antisense oligonucleotide (ASO) in mice with dietary NAFLD and a genetic deficiency of pRb or its downstream effector E2F1, as well as in primary human hepatocytes.
Results
T39 interacts with pRb via its C-terminal TPR domain and promotes its proteasomal degradation. In female mice, T39 deficiency reduces the mRNA of lipogenic genes, especially Pnpla3, in a pRb- and E2F1-dependent manner. In contrast, in male mice, T39 deficiency results in a much smaller reduction in lipogenic gene expression that is independent of pRb/E2F1. T39 also interacts with VAPB via an N-terminal FFAT motif and stabilizes the interaction of VAPB with SCAP. Ovariectomy abolishes the effect of T39 knockdown on the hepatic pRb/E2F1/Pnpla3 axis. In both sexes T39 knockdown reduces SCAP independently of pRb. In primary human hepatocytes, T39 knockdown reduces expression of PNPLA3 and other lipogenic genes in women but not men.
Conclusions
We have uncovered a conserved sexual dimorphism in the regulation of hepatic lipogenic genes, with effects of T39 mediated through pRb/E2F1 in females and VAPB/SCAP in both sexes. T39 inhibition could be a novel strategy to downregulate PNPLA3 and treat NAFLD in women.
Lay summary
In females, the protein TTC39B degrades a tumor suppressor in the liver to promote the synthesis of new fat and the expression of a major genetic risk factor for non-alcoholic fatty liver disease. TTC39B is a potential therapeutic target for non-alcoholic fatty liver disease, especially in women.


































 京公网安备 11010802027423号
京公网安备 11010802027423号