Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2021-09-30 , DOI: 10.1016/j.cej.2021.132686
Hugo Olvera-Vargas 1, 2 , Zuxin Wang 1, 3 , Jianxiong Xu 1 , Olivier Lefebvre 1
|
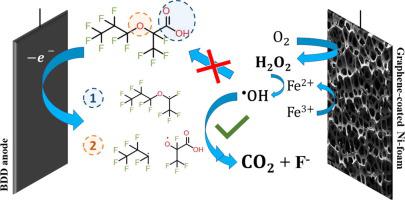
Perfluoroalkyl substances (PFAS) are well-recognized water pollutants. Even though most studies have traditionally focused on long-chain PFAS, their progressive replacement by short-chain PFAS has made the latter a new motive of concern. For example, short-chain hexafluoropropylene oxide dimer acid (known as GenX) was introduced as a supposedly sustainable substitute for toxic perfluorooctanic acid, but is now being detected in several aquatic environments around the world, with various reports evidencing its harmful effects. In this work, we investigated the degradation/mineralization of GenX by electro-Fenton (EF) using a graphene-Ni-foam (Gr-Ni-foam) cathode paired with a boron doped diamond (BDD) anode. It was found that homogeneous •OH radicals formed by EF showed limited reactivity with the GenX molecule itself. Instead, GenX degradation was initiated by direct electron transfer at the BDD surface, and then homogeneous •OH continued and enhanced the degradation/mineralization process. In contrast, Pt and fluorine doped tin oxide (FTO) anodes did not favor GenX degradation. A synergy between EF and BDD oxidation achieved 92.2 ± 1.0% of GenX mineralization after 6 h of treatment at 16 mA cm-2, vs. only 9.2 ± 0.1% by EF alone and 73.6 ± 6.2% with BDD alone. Based on the degradation by-products detected by high performance liquid chromatography-mass spectrometry (HPLC-MS), a mineralization pathway for GenX is proposed, involving two different routes initiated by electron transfer at the carboxylic and ether groups of the GenX molecule.
中文翻译:

通过配对石墨烯涂层镍泡沫和掺硼金刚石电极协同降解 GenX(六氟环氧丙烷二聚酸)
全氟烷基物质 (PFAS) 是公认的水污染物。尽管大多数研究传统上都集中在长链 PFAS 上,但它们逐渐被短链 PFAS 取代使后者成为关注的新动机。例如,短链六氟环氧丙烷二聚酸(称为 GenX)被引入作为有毒全氟辛酸的可持续替代品,但现在在世界各地的几个水生环境中被检测到,各种报告证明其有害影响。在这项工作中,我们使用石墨烯-镍泡沫 (Gr-Ni-泡沫) 阴极与掺硼金刚石 (BDD) 阳极配对,研究了电芬顿 (EF) 对 GenX 的降解/矿化。发现同质的•EF 形成的 OH 自由基与 GenX 分子本身的反应性有限。相反,GenX 降解是由 BDD 表面的直接电子转移引发的,然后均质• OH 继续并增强了降解/矿化过程。相比之下,Pt 和掺氟氧化锡 (FTO) 阳极不利于 GenX 降解。在 16 mA cm -2下处理 6 小时后,EF 和 BDD 氧化之间的协同作用实现了 92.2 ± 1.0% 的 GenX 矿化,而单独使用 EF 时仅为 9.2 ± 0.1%,单独使用 BDD 时为 73.6 ± 6.2%。基于高效液相色谱-质谱 (HPLC-MS) 检测到的降解副产物,提出了 GenX 的矿化途径,涉及由 GenX 分子的羧基和醚基团处的电子转移引发的两种不同途径。

































 京公网安备 11010802027423号
京公网安备 11010802027423号