当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enabling N-to-C Ser/Thr Ligation for Convergent Protein Synthesis via Combining Chemical Ligation Approaches
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2016-08-15 , DOI: 10.1021/jacs.6b04238 Chi Lung Lee 1 , Han Liu 1 , Clarence T. T. Wong 1 , Hoi Yee Chow 1 , Xuechen Li 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2016-08-15 , DOI: 10.1021/jacs.6b04238 Chi Lung Lee 1 , Han Liu 1 , Clarence T. T. Wong 1 , Hoi Yee Chow 1 , Xuechen Li 1
Affiliation
|
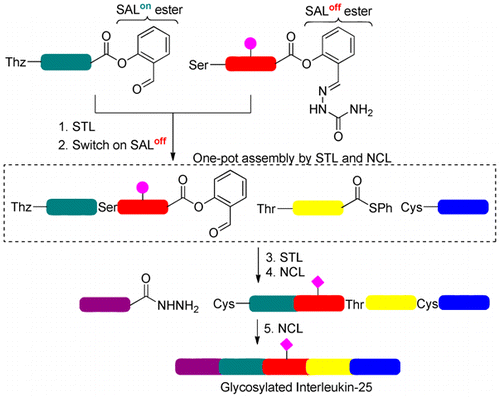
In this article, Ser/Thr ligation(on/off) has been realized to enable N-to-C successive peptide ligations using a salicylaldehyde semicarbazone (SAL(off)) group by in situ activation with pyruvic acid of the peptide SAL(off) ester into the peptide salicylaldehyde (SAL(on)) ester. In addition, a peptide with a C-terminal thioester and N-terminal Ser or Thr as the middle peptide segment can undergo one-pot Ser/Thr ligation and native chemical ligation in the N-to-C direction. The utility of this combined ligation strategy in the N-to-C direction has been showcased through the convergent assembly of a human cytokine protein sequence, GlcNAcylated interleukin-25.
中文翻译:

通过结合化学连接方法实现 N-to-C Ser/Thr 连接以进行聚合蛋白质合成
在本文中,Ser/Thr ligation(on/off) 实现了使用水杨醛缩氨基脲 (SAL(off)) 基团通过肽 SAL(off) 的丙酮酸原位激活实现 N 到 C 连续肽连接。 ) 酯转化为肽水杨醛 (SAL(on)) 酯。此外,具有 C 端硫酯和 N 端 Ser 或 Thr 作为中间肽段的肽可以在 N 到 C 方向上进行单锅 Ser/Thr 连接和天然化学连接。这种组合连接策略在 N 到 C 方向的效用已通过人类细胞因子蛋白序列 GlcNAcylated interleukin-25 的会聚组装得到展示。
更新日期:2016-08-15
中文翻译:

通过结合化学连接方法实现 N-to-C Ser/Thr 连接以进行聚合蛋白质合成
在本文中,Ser/Thr ligation(on/off) 实现了使用水杨醛缩氨基脲 (SAL(off)) 基团通过肽 SAL(off) 的丙酮酸原位激活实现 N 到 C 连续肽连接。 ) 酯转化为肽水杨醛 (SAL(on)) 酯。此外,具有 C 端硫酯和 N 端 Ser 或 Thr 作为中间肽段的肽可以在 N 到 C 方向上进行单锅 Ser/Thr 连接和天然化学连接。这种组合连接策略在 N 到 C 方向的效用已通过人类细胞因子蛋白序列 GlcNAcylated interleukin-25 的会聚组装得到展示。































 京公网安备 11010802027423号
京公网安备 11010802027423号