当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Scalable Asymmetric Synthesis of MK-8998, a T-Type Calcium Channel Antagonist
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-09-28 , DOI: 10.1021/acs.joc.1c01795 Yong-Li Zhong 1 , Jeffrey C Moore 1 , Michael Shevlin 1 , C Scott Shultz 1 , Birgit Kosjek 1 , Yonggang Chen 1 , Jacob M Janey 1 , Lushi Tan 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-09-28 , DOI: 10.1021/acs.joc.1c01795 Yong-Li Zhong 1 , Jeffrey C Moore 1 , Michael Shevlin 1 , C Scott Shultz 1 , Birgit Kosjek 1 , Yonggang Chen 1 , Jacob M Janey 1 , Lushi Tan 1
Affiliation
|
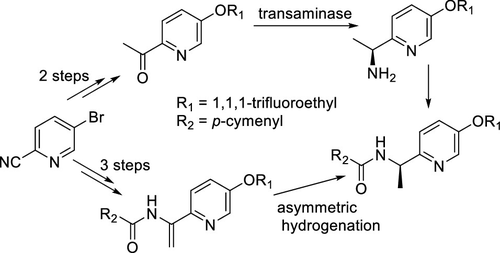
Two scalable and efficient synthetic routes for the synthesis of a T-type calcium channel antagonist MK-8998 were developed from a simple pyridine building block. The key step to set the stereochemistry relied on either chiral rhodium catalyst-mediated asymmetric hydrogenation of an enamide or transamination of an arylketone that provided the corresponding product in high enantioselectivity and high yield.
中文翻译:

T 型钙通道拮抗剂 MK-8998 的可扩展不对称合成
从简单的吡啶构建块开发了两种用于合成 T 型钙通道拮抗剂 MK-8998 的可扩展且有效的合成路线。设置立体化学的关键步骤依赖于手性铑催化剂介导的烯酰胺不对称氢化或芳基酮的氨基转移,从而以高对映选择性和高产率提供相应的产物。
更新日期:2021-09-28
中文翻译:

T 型钙通道拮抗剂 MK-8998 的可扩展不对称合成
从简单的吡啶构建块开发了两种用于合成 T 型钙通道拮抗剂 MK-8998 的可扩展且有效的合成路线。设置立体化学的关键步骤依赖于手性铑催化剂介导的烯酰胺不对称氢化或芳基酮的氨基转移,从而以高对映选择性和高产率提供相应的产物。































 京公网安备 11010802027423号
京公网安备 11010802027423号