当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Asymmetric Alkynylation of 3,4-Dihydro-β-carbolinium Ions Enables Collective Total Syntheses of Indole Alkaloids
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2021-09-28 , DOI: 10.1002/anie.202112383 Lixin Liang 1 , Shiqiang Zhou 1 , Wei Zhang 1 , Rongbiao Tong 1, 2
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2021-09-28 , DOI: 10.1002/anie.202112383 Lixin Liang 1 , Shiqiang Zhou 1 , Wei Zhang 1 , Rongbiao Tong 1, 2
Affiliation
|
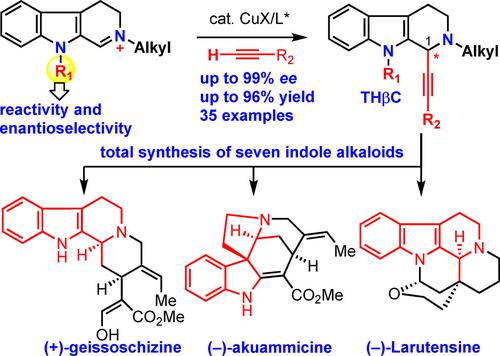
Catalytic asymmetric alkynylation of 3,4-dihydro-β-carbolinium ions was developed for the versatile synthesis of enantiomerically enriched C1-alkynyl tetrahydro-β-carbolines, the utility of which was demonstrated by concise collective total syntheses of the seven indole alkaloids harmicine, geissoschizol, geissoschizine, akuammicine, desethyleburnamonine, eburnamonine, and larutensine within 10 steps (see scheme).

中文翻译:

3,4-二氢-β-咔啉离子的催化不对称炔化使吲哚生物碱的集体全合成成为可能
开发了 3,4-二氢-β-咔啉离子的催化不对称炔化作用,用于合成富含对映异构体的 C1-炔基四氢-β-咔啉,其效用已通过七种吲哚生物碱甘草碱的简明集体全合成证明, geissoschizol、geissoschizine、akuammicine、desethyleburnamonine、eburnamonine 和 larutensine 在 10 个步骤内(见方案)。

更新日期:2021-11-08

中文翻译:

3,4-二氢-β-咔啉离子的催化不对称炔化使吲哚生物碱的集体全合成成为可能
开发了 3,4-二氢-β-咔啉离子的催化不对称炔化作用,用于合成富含对映异构体的 C1-炔基四氢-β-咔啉,其效用已通过七种吲哚生物碱甘草碱的简明集体全合成证明, geissoschizol、geissoschizine、akuammicine、desethyleburnamonine、eburnamonine 和 larutensine 在 10 个步骤内(见方案)。



















































 京公网安备 11010802027423号
京公网安备 11010802027423号