Chem Catalysis ( IF 11.5 ) Pub Date : 2021-09-28 , DOI: 10.1016/j.checat.2021.09.004 Changcheng Wei 1, 2 , Jinzhe Li 1 , Kuo Yang 1 , Qijun Yu 1 , Shu Zeng 1, 2 , Zhongmin Liu 1, 2
|
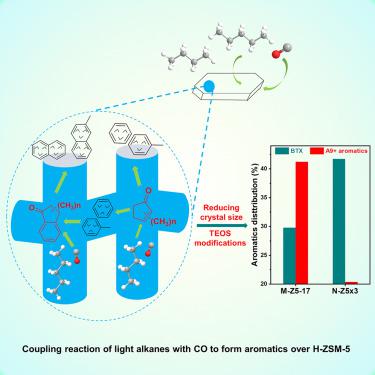
The aromatization of light alkanes (C4-C6) is an important value-added reaction. However, the yield of aromatics is always limited by the simultaneous generation of small alkanes (CH4 and C2H6) due to H/C balance. Herein, we demonstrate that the introduction of CO into light alkanes over zeolites significantly enhanced aromatic selectivity and an aromatics selectivity of 85% was achieved in the case of cyclopentane and CO coupling reaction over H-ZSM-5. Methyl-substituted cyclopentenones were observed and considered as the most important intermediates for aromatics formation. Multiple characterizations revealed the coupling mechanism: (1) CO inserts into carbenium ions to form acylium cations, (2) acylium cations react with olefins to generate unsaturated ketones, (3) unsaturated ketones cyclize to methyl-substituted cyclopentenones, (4) methyl-substituted cyclopentenones convert to monocytic aromatics. Methyl-substituted indanones were also discovered causing the generation of binuclear aromatics, such as naphthalene. Nano-sized ZSM-5 and TEOS modifications were applied to enhance the BTX selectivity.
中文翻译:

轻质烷烃与CO在酸性沸石上偶联反应的芳构化机理:环戊烯酮作为关键中间体
轻质烷烃(C 4 -C 6)的芳构化是一个重要的增值反应。然而,芳烃的产率总是受到小烷烃(CH 4和 C 2 H 6) 由于 H/C 平衡。在此,我们证明在沸石上将 CO 引入轻质烷烃显着提高了芳烃选择性,并且在环戊烷和 CO 偶联反应的情况下,通过 H-ZSM-5 实现了 85% 的芳烃选择性。甲基取代的环戊烯酮被观察到并被认为是形成芳烃的最重要的中间体。多个表征揭示了耦合机制:(1)CO 插入碳正离子形成酰基阳离子,(2)酰基阳离子与烯烃反应生成不饱和酮,(3)不饱和酮环化为甲基取代的环戊烯酮,(4)甲基-取代的环戊烯酮转化为单核芳烃。还发现甲基取代的茚满酮会导致双核芳烃的产生,例如萘。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号