当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of 5-{4-[(7-Ethyl-6-oxo-5,6-dihydro-1,5-naphthyridin-3-yl)methyl]piperazin-1-yl}-N-methylpyridine-2-carboxamide (AZD5305): A PARP1–DNA Trapper with High Selectivity for PARP1 over PARP2 and Other PARPs
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2021-09-27 , DOI: 10.1021/acs.jmedchem.1c01012 Jeffrey W Johannes 1 , Amber Balazs 1 , Derek Barratt 2 , Michal Bista 2 , Matthew D Chuba 1 , Sabina Cosulich 3 , Susan E Critchlow 4 , Sébastien L Degorce 1 , Paolo Di Fruscia 2 , Scott D Edmondson 1 , Kevin Embrey 2 , Stephen Fawell 5 , Avipsa Ghosh 1 , Sonja J Gill 6 , Anders Gunnarsson 7 , Sudhir M Hande 1 , Tom D Heightman 8 , Paul Hemsley 2 , Giuditta Illuzzi 4 , Jordan Lane 2 , Carrie Larner 6 , Elisabetta Leo 4 , Lina Liu 9 , Andrew Madin 2 , Scott Martin 10 , Lisa McWilliams 2 , Mark J O'Connor 4 , Jonathan P Orme 2 , Fiona Pachl 11 , Martin J Packer 12 , Xiaohui Pei 9 , Andrew Pike 10 , Marianne Schimpl 2 , Hongyao She 9 , Anna D Staniszewska 4 , Verity Talbot 2 , Elizabeth Underwood 2 , Jeffrey G Varnes 1 , Lin Xue 9 , Tieguang Yao 9 , Ke Zhang 9 , Andrew X Zhang 11 , Xiaolan Zheng 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2021-09-27 , DOI: 10.1021/acs.jmedchem.1c01012 Jeffrey W Johannes 1 , Amber Balazs 1 , Derek Barratt 2 , Michal Bista 2 , Matthew D Chuba 1 , Sabina Cosulich 3 , Susan E Critchlow 4 , Sébastien L Degorce 1 , Paolo Di Fruscia 2 , Scott D Edmondson 1 , Kevin Embrey 2 , Stephen Fawell 5 , Avipsa Ghosh 1 , Sonja J Gill 6 , Anders Gunnarsson 7 , Sudhir M Hande 1 , Tom D Heightman 8 , Paul Hemsley 2 , Giuditta Illuzzi 4 , Jordan Lane 2 , Carrie Larner 6 , Elisabetta Leo 4 , Lina Liu 9 , Andrew Madin 2 , Scott Martin 10 , Lisa McWilliams 2 , Mark J O'Connor 4 , Jonathan P Orme 2 , Fiona Pachl 11 , Martin J Packer 12 , Xiaohui Pei 9 , Andrew Pike 10 , Marianne Schimpl 2 , Hongyao She 9 , Anna D Staniszewska 4 , Verity Talbot 2 , Elizabeth Underwood 2 , Jeffrey G Varnes 1 , Lin Xue 9 , Tieguang Yao 9 , Ke Zhang 9 , Andrew X Zhang 11 , Xiaolan Zheng 1
Affiliation
|
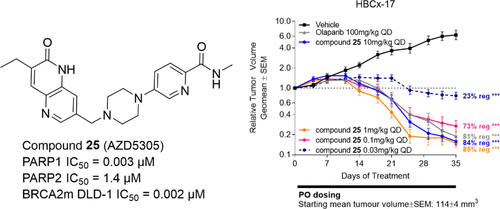
Poly-ADP-ribose-polymerase (PARP) inhibitors have achieved regulatory approval in oncology for homologous recombination repair deficient tumors including BRCA mutation. However, some have failed in combination with first-line chemotherapies, usually due to overlapping hematological toxicities. Currently approved PARP inhibitors lack selectivity for PARP1 over PARP2 and some other 16 PARP family members, and we hypothesized that this could contribute to toxicity. Recent literature has demonstrated that PARP1 inhibition and PARP1–DNA trapping are key for driving efficacy in a BRCA mutant background. Herein, we describe the structure- and property-based design of 25 (AZD5305), a potent and selective PARP1 inhibitor and PARP1–DNA trapper with excellent in vivo efficacy in a BRCA mutant HBCx-17 PDX model. Compound 25 is highly selective for PARP1 over other PARP family members, with good secondary pharmacology and physicochemical properties and excellent pharmacokinetics in preclinical species, with reduced effects on human bone marrow progenitor cells in vitro.
中文翻译:

发现5-{4-[(7-乙基-6-氧代-5,6-二氢-1,5-萘啶-3-基)甲基]哌嗪-1-基}-N-甲基吡啶-2-甲酰胺( AZD5305):一种 PARP1–DNA 捕获剂,对 PARP1 的选择性高于 PARP2 和其他 PARP
聚 ADP 核糖聚合酶 (PARP) 抑制剂已获得肿瘤学监管部门的批准,用于同源重组修复缺陷肿瘤,包括 BRCA 突变。然而,一些药物与一线化疗联合治疗失败,通常是由于重叠的血液学毒性。目前批准的 PARP 抑制剂对 PARP1 的选择性相对于 PARP2 和其他一些 16 个 PARP 家族成员缺乏选择性,我们假设这可能会导致毒性。最近的文献表明,PARP1 抑制和 PARP1-DNA 捕获是在 BRCA 突变背景下提高疗效的关键。在此,我们描述了25 (AZD5305) 的基于结构和性质的设计,25 是一种有效的选择性 PARP1 抑制剂和 PARP1-DNA 捕获剂,在 BRCA 突变体 HBCx-17 PDX 模型中具有出色的体内功效。与其他 PARP 家族成员相比,化合物25对 PARP1 具有高度选择性,具有良好的二级药理学和理化性质,在临床前物种中具有优异的药代动力学,体外对人骨髓祖细胞的影响较小。
更新日期:2021-10-14
中文翻译:

发现5-{4-[(7-乙基-6-氧代-5,6-二氢-1,5-萘啶-3-基)甲基]哌嗪-1-基}-N-甲基吡啶-2-甲酰胺( AZD5305):一种 PARP1–DNA 捕获剂,对 PARP1 的选择性高于 PARP2 和其他 PARP
聚 ADP 核糖聚合酶 (PARP) 抑制剂已获得肿瘤学监管部门的批准,用于同源重组修复缺陷肿瘤,包括 BRCA 突变。然而,一些药物与一线化疗联合治疗失败,通常是由于重叠的血液学毒性。目前批准的 PARP 抑制剂对 PARP1 的选择性相对于 PARP2 和其他一些 16 个 PARP 家族成员缺乏选择性,我们假设这可能会导致毒性。最近的文献表明,PARP1 抑制和 PARP1-DNA 捕获是在 BRCA 突变背景下提高疗效的关键。在此,我们描述了25 (AZD5305) 的基于结构和性质的设计,25 是一种有效的选择性 PARP1 抑制剂和 PARP1-DNA 捕获剂,在 BRCA 突变体 HBCx-17 PDX 模型中具有出色的体内功效。与其他 PARP 家族成员相比,化合物25对 PARP1 具有高度选择性,具有良好的二级药理学和理化性质,在临床前物种中具有优异的药代动力学,体外对人骨髓祖细胞的影响较小。






























 京公网安备 11010802027423号
京公网安备 11010802027423号