Letters in Drug Design & Discovery ( IF 1.2 ) Pub Date : 2021-06-30 , DOI: 10.2174/1570180817999201211193151 Sergey S. Patrushev 1 , Lyubov G. Burova 2 , Anna A. Shtro 3 , Tatyana V. Rybalova 4 , Dmitry S. Baev 4 , Il’ya V. Shirokikh 2 , Alexander N. Evstropov 2 , Elvira E. Shults 4
|
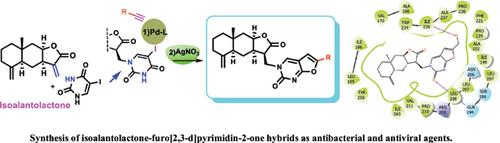
Background: Natural sesquiterpene lactones are an important class of heterocyclic compounds in drug discovery since they possess a wide range of biological properties, including antibacterial activity.
Objective: The objective of this study was to synthesize isoalantolactone derivatives with a furo[2,3-d] pyrimidin-2-оne moiety, and to evaluate their antibacterial and antiviral activity.
Methods: The Sonogashira cross-coupling and subsequent Ag-catalyzed cyclization reactions were used forthe synthesis. The antibacterial activity and the ability to inhibit biofilms formation on E. coli, S. aureus, A. viscosus, P. aeruginosa, and E. faecalis bacterial strains were evaluated in this study. A study of the molecular interactions of new compounds with the multiple virulence factor regulators was performed using docking simulations. The anti-viral activity against influenza A virus and human orthopneumovirus H-2А was also studied.
Results: The in vitro anti-bacterial activity for compound 4 (MIC = 58.33 ± 4.41 μg/mL) concerning E. coli and compound 5 (MIC = 96.5 ± 3.25 μg/mL) against A. viscosus and the inhibition of biofilm formation for compounds 2, 4, and 5 on E. coli, S. aureus, P. aeruginosa, and E. faecalis bacterial strains, have been of interest for the search of improved anti-microbial agents. Compound 3 possessed antiviral activity against human orthopneumovirus H-2А with SI >33. The activity of the new type of hybrid compounds is dependent on the substituent in the 6th position of the furo[2,3-d] pyrimidin-2-one fragment.
Conclusion: The decoration of isoalantolactone with a furo[2,3-d]pyrimidin-2-one fragment led to the development of antiviral and antimicrobial agents. Due to the antimicrobial activity, pyridine-4- yl substituted isoalantolactone-furopyrimidinone hybrid is considered as a candidate compound to participate in further research.
中文翻译:

导致有效抗菌和抗病毒化合物的异戊内酯的改性
背景:天然倍半萜内酯是药物发现中一类重要的杂环化合物,因为它们具有广泛的生物学特性,包括抗菌活性。
目的:本研究的目的是合成具有 furo[2,3-d] pyrimidin-2-one 部分的异丙内酯衍生物,并评估其抗菌和抗病毒活性。
方法:采用Sonogashira交叉偶联和随后的银催化环化反应合成。本研究评估了大肠杆菌、金黄色葡萄球菌、粘菌、铜绿假单胞菌和粪肠球菌的抗菌活性和抑制生物膜形成的能力。使用对接模拟对新化合物与多毒力因子调节剂的分子相互作用进行了研究。还研究了针对甲型流感病毒和人类正肺病毒 H-2A 的抗病毒活性。
结果:化合物 4 (MIC = 58.33 ± 4.41 μg/mL) 对大肠杆菌的体外抗菌活性和化合物 5 (MIC = 96.5 ± 3.25 μg/mL) 对 A. viscosus 的体外抗菌活性和生物膜形成的抑制作用大肠杆菌、金黄色葡萄球菌、铜绿假单胞菌和粪肠球菌细菌菌株上的化合物 2、4 和 5 对寻找改进的抗微生物剂很感兴趣。化合物 3 对人正肺病毒 H-2- 具有抗病毒活性,SI > 33。新型杂化化合物的活性取决于呋喃[2,3-d]嘧啶-2-one片段第6位的取代基。
结论:异羊毛脂内酯与呋喃[2,3-d]嘧啶-2-one 片段的修饰导致了抗病毒和抗微生物剂的开发。由于抗微生物活性,吡啶-4-基取代的异戊内酯-呋喃嘧啶酮杂化物被认为是参与进一步研究的候选化合物。








































 京公网安备 11010802027423号
京公网安备 11010802027423号