当前位置:
X-MOL 学术
›
Acta Cryst. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis, spectroscopic characterization, structural studies, thermal analysis and molecular docking of N-(2-methyl-5-nitrophenyl)-4-(pyridin-2-yl)pyrimidin-2-amine, a precursor for drug design against chronic myeloid leukemia
Acta Crystallographica Section C ( IF 0.7 ) Pub Date : 2021-09-23 , DOI: 10.1107/s2053229621009487 Rodolfo Moreno-Fuquen 1 , Kevin Arango-Daraviña 1 , Alan R Kennedy 2
Acta Crystallographica Section C ( IF 0.7 ) Pub Date : 2021-09-23 , DOI: 10.1107/s2053229621009487 Rodolfo Moreno-Fuquen 1 , Kevin Arango-Daraviña 1 , Alan R Kennedy 2
Affiliation
|
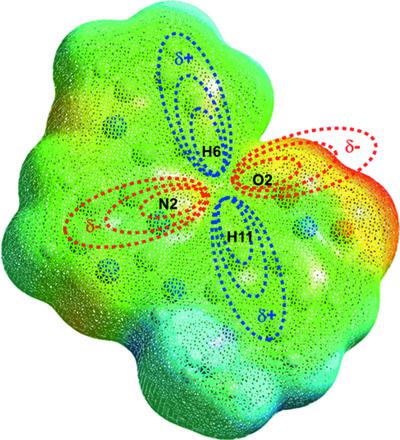
The synthesis, crystal structure and spectroscopic and electronic properties of N-(2-methyl-5-nitrophenyl)-4-(pyridin-2-yl)pyrimidin-2-amine (NPPA), C16H13N5O2, a potential template for drug design against chronic myelogenous leukemia (CML), is reported. The design and construction of the target molecule were carried out starting from the guanidinium nitrate salt (previously synthesized) and the corresponding enaminone. X-ray diffraction analysis and a study of the Hirshfeld surfaces revealed important interactions between the nitro-group O atoms and the H atoms of the pyridine and pyrimidine rings. A crystalline ordering in layers, by the stacking of rings through interactions of the π–π type, was observed and confirmed by a study of the shape-index surfaces and dispersion energy calculations. Quantitative electrostatic potential studies revealed the most positive value of the molecule on regions close to the N—H groups (34.8 kcal mol−1); nevertheless, steric impediments and the planarity of the molecule do not allow the formation of hydrogen bonds from this group. This interaction is however activated when the molecule takes on a new extended conformation in the active pocket of the enzyme kinase (PDB ID 2hyy), interacting with protein residues that are fundamental in the inhibition process of CML. The most negative values of the molecule are seen in regions close to the nitro group (−35.4 and −34.0 kcal mol−1). A molecular docking study revealed an energy affinity of ΔG = −10.3 kcal mol−1 for NPPA which, despite not having a more negative value than the control molecule (Imatinib; ΔG = −12.8 kcal mol−1), shows great potential to be used as a template for new drugs against CML.
中文翻译:

N-(2-methyl-5-nitrophenyl)-4-(pyridin-2-yl)pyrimidin-2-amine 的合成、光谱表征、结构研究、热分析和分子对接,一种用于慢性粒细胞白血病药物设计的前体
N- (2-methyl-5-nitrophenyl)-4-(pyridin-2-yl)pyrimidin-2-amine ( NPPA ), C 16 H 13 N 5 O 2的合成、晶体结构、光谱和电子性质据报道,这是一种针对慢性粒细胞白血病 (CML) 的药物设计的潜在模板。目标分子的设计和构建从硝酸胍盐(先前合成)和相应的烯胺酮开始。X 射线衍射分析和对 Hirshfeld 表面的研究揭示了硝基 O 原子与吡啶和嘧啶环的 H 原子之间的重要相互作用。通过对形状指数表面和色散能计算的研究,观察并证实了通过 π-π 型相互作用堆叠环而形成的层状晶体有序性。定量静电势研究揭示了分子在靠近 NH 基团(34.8 kcal mol -1); 然而,空间位阻和分子的平面性不允许从该基团形成氢键。然而,当分子在酶激酶的活性口袋 (PDB ID 2hyy) 中呈现新的扩展构象时,这种相互作用被激活,与 CML 抑制过程中的基本蛋白质残基相互作用。在靠近硝基的区域(-35.4 和-34.0 kcal mol -1)可以看到分子的最负值。甲分子对接研究揭示Δ的能量亲和力ģ = -10.3千卡摩尔-1为NPPA其中,尽管不具有比对照分子更负的值(伊马替尼;Δ ģ = -12.8千卡摩尔-1),显示出用作抗 CML 新药模板的巨大潜力。
更新日期:2021-10-06
中文翻译:

N-(2-methyl-5-nitrophenyl)-4-(pyridin-2-yl)pyrimidin-2-amine 的合成、光谱表征、结构研究、热分析和分子对接,一种用于慢性粒细胞白血病药物设计的前体
N- (2-methyl-5-nitrophenyl)-4-(pyridin-2-yl)pyrimidin-2-amine ( NPPA ), C 16 H 13 N 5 O 2的合成、晶体结构、光谱和电子性质据报道,这是一种针对慢性粒细胞白血病 (CML) 的药物设计的潜在模板。目标分子的设计和构建从硝酸胍盐(先前合成)和相应的烯胺酮开始。X 射线衍射分析和对 Hirshfeld 表面的研究揭示了硝基 O 原子与吡啶和嘧啶环的 H 原子之间的重要相互作用。通过对形状指数表面和色散能计算的研究,观察并证实了通过 π-π 型相互作用堆叠环而形成的层状晶体有序性。定量静电势研究揭示了分子在靠近 NH 基团(34.8 kcal mol -1); 然而,空间位阻和分子的平面性不允许从该基团形成氢键。然而,当分子在酶激酶的活性口袋 (PDB ID 2hyy) 中呈现新的扩展构象时,这种相互作用被激活,与 CML 抑制过程中的基本蛋白质残基相互作用。在靠近硝基的区域(-35.4 和-34.0 kcal mol -1)可以看到分子的最负值。甲分子对接研究揭示Δ的能量亲和力ģ = -10.3千卡摩尔-1为NPPA其中,尽管不具有比对照分子更负的值(伊马替尼;Δ ģ = -12.8千卡摩尔-1),显示出用作抗 CML 新药模板的巨大潜力。






































 京公网安备 11010802027423号
京公网安备 11010802027423号