当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Diastereo- and Enantioselective Vinylogous Mannich Reaction of Alkylidenepyrazolones to Isatin-Derived Ketimines
Organic Letters ( IF 4.9 ) Pub Date : 2021-09-23 , DOI: 10.1021/acs.orglett.1c02571 Laura Carceller-Ferrer 1 , Carlos Vila 1 , Gonzalo Blay 1 , M Carmen Muñoz 2 , José R Pedro 1
Organic Letters ( IF 4.9 ) Pub Date : 2021-09-23 , DOI: 10.1021/acs.orglett.1c02571 Laura Carceller-Ferrer 1 , Carlos Vila 1 , Gonzalo Blay 1 , M Carmen Muñoz 2 , José R Pedro 1
Affiliation
|
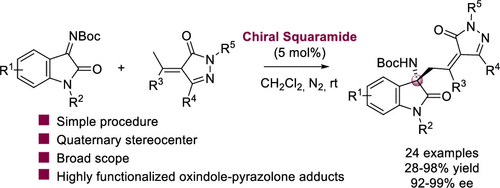
A valuable organocatalytic vinylogous Mannich reaction between alkylidenepyrazolones and isatin-derived ketimines has been successfully established. Squaramide organocatalyst, prepared from quinine, catalyzed the diastereo- and enantioselective vinylogous Mannich addition, affording a range of aminooxindole-pyrazolone adducts (24 examples) with excellent outcomes: up to 98% yield with complete diastereoselectivity and excellent enantioselectivity (up to 99% ee). Additionally, different synthetic transformations were performed with the chiral pyrazolone-oxindole adducts.
中文翻译:

亚烷基吡唑啉酮与靛红衍生的酮亚胺的催化非对映选择性和对映选择性乙烯基曼尼希反应
亚烷基吡唑啉酮和靛红衍生的酮亚胺之间有价值的有机催化乙烯基曼尼希反应已经成功建立。由奎宁制备的方酸酰胺有机催化剂催化非对映选择性和对映选择性乙烯基曼尼希加成,提供一系列氨基吲哚-吡唑啉酮加合物(24 个例子),结果优异:高达 98% 的收率,完全非对映选择性和出色的对映选择性(高达 99% ee )。此外,使用手性吡唑啉酮-羟吲哚加合物进行了不同的合成转化。
更新日期:2021-10-01
中文翻译:

亚烷基吡唑啉酮与靛红衍生的酮亚胺的催化非对映选择性和对映选择性乙烯基曼尼希反应
亚烷基吡唑啉酮和靛红衍生的酮亚胺之间有价值的有机催化乙烯基曼尼希反应已经成功建立。由奎宁制备的方酸酰胺有机催化剂催化非对映选择性和对映选择性乙烯基曼尼希加成,提供一系列氨基吲哚-吡唑啉酮加合物(24 个例子),结果优异:高达 98% 的收率,完全非对映选择性和出色的对映选择性(高达 99% ee )。此外,使用手性吡唑啉酮-羟吲哚加合物进行了不同的合成转化。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号