Journal of the Taiwan Institute of Chemical Engineers ( IF 5.5 ) Pub Date : 2021-09-23 , DOI: 10.1016/j.jtice.2021.09.012
Xiaoli Wei 1 , Hong Yang 1 , Bing Liu 1 , Houlin Yu 1 , Chuanzhao Wang 1 , Sen Wu 1 , Zhongsheng Jia 1 , Wenfeng Han 1
|
Background
Hydrofluorocarbons (HFCs) are potent greenhouse gasses with high global warming potential and the emission of which is strictly regulated by Kigali amendment.
Methods
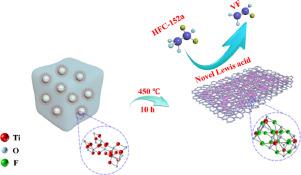
In this paper, titanium oxyfluoride (TiOF2) was firstly synthesized by simple solution combustion synthesis in the absence of highly corrosive HF, among which calcination played an important role on the phase of the products. When the precursor was calcinated at 450 °C, pure TiOF2 could be obtained.
Significant findings
The pyrolysis of HFCs results showed that the 1,1-difluoroethane (HFC-152a) conversion over TiOF2 is about 53%, superior than TiO2 (40%) due to the enhanced Lewis acidity. Even compared with traditional metal fluoride catalysts (AlF3 and MgF2), TiOF2 held higher stability within 35 h, contributing to the suitable Lewis acidic sites. The formation mechanism of the Lewis acidity was systematically investigated: on one hand, F species made the binding energy of Ti spesies shift to high regions to enhance the Lewis acid strength of Ti species; on the other hand, more oxygen vacancies were generated by the introduction of F, which could act as the Lewis acid sites as well. Hence, this study provides a promising and potential candidate for the catalytic pyrolysis of HFCs.
中文翻译:

含氧空位的氟氧化钛作为含氟温室气体热解制备氢氟烯烃的新型催化剂的合成
背景
氢氟碳化合物 (HFC) 是具有高全球变暖潜势的强效温室气体,其排放受到基加利修正案的严格管制。
方法
在本文中,氟氧化钛(TiOF 2)首先在没有强腐蚀性HF的情况下通过简单的溶液燃烧合成合成,其中煅烧对产物的相态起着重要作用。当前体在 450°C 下煅烧时,可以获得纯 TiOF 2。
重要发现
HFCs 的热解结果表明 1,1-二氟乙烷 (HFC-152a) 在 TiOF 2上的转化率约为 53%,由于增强的路易斯酸度,优于 TiO 2 (40%)。即使与传统的金属氟化物催化剂(AlF 3和 MgF 2)相比,TiOF 2在 35 小时内保持更高的稳定性,有助于形成合适的路易斯酸性位点。系统研究了路易斯酸的形成机制:一方面,F物种使Ti物种的结合能向高区域移动,从而增强了Ti物种的路易斯酸强度;另一方面,F 的引入产生了更多的氧空位,F 也可以作为路易斯酸位。因此,这项研究为 HFCs 的催化热解提供了一个有前途的潜在候选者。































 京公网安备 11010802027423号
京公网安备 11010802027423号