当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
JNJ-67569762, A 2-Aminotetrahydropyridine-Based Selective BACE1 Inhibitor Targeting the S3 Pocket: From Discovery to Clinical Candidate
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2021-09-23 , DOI: 10.1021/acs.jmedchem.1c00935 Frederik J R Rombouts 1 , Ken-Ichi Kusakabe 2 , Richard Alexander 3 , Nigel Austin 1 , Herman Borghys 1 , Michel De Cleyn 1 , Deborah Dhuyvetter 1 , Harrie J M Gijsen 1 , Brian Hrupka 1 , Tom Jacobs 1 , Soufyan Jerhaoui 1 , Lieve Lammens 1 , Laurent Leclercq 1 , Koichi Tsubone 2 , Tatsuhiko Ueno 2 , Kenji Morimoto 2 , Shunsuke Einaru 2 , Hirokazu Sumiyoshi 2 , An Van den Bergh 1 , Ann Vos 1 , Michel Surkyn 1 , Ard Teisman 1 , Diederik Moechars 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2021-09-23 , DOI: 10.1021/acs.jmedchem.1c00935 Frederik J R Rombouts 1 , Ken-Ichi Kusakabe 2 , Richard Alexander 3 , Nigel Austin 1 , Herman Borghys 1 , Michel De Cleyn 1 , Deborah Dhuyvetter 1 , Harrie J M Gijsen 1 , Brian Hrupka 1 , Tom Jacobs 1 , Soufyan Jerhaoui 1 , Lieve Lammens 1 , Laurent Leclercq 1 , Koichi Tsubone 2 , Tatsuhiko Ueno 2 , Kenji Morimoto 2 , Shunsuke Einaru 2 , Hirokazu Sumiyoshi 2 , An Van den Bergh 1 , Ann Vos 1 , Michel Surkyn 1 , Ard Teisman 1 , Diederik Moechars 1
Affiliation
|
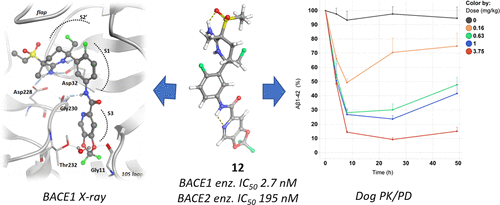
The discovery of a novel 2-aminotetrahydropyridine class of BACE1 inhibitors is described. Their pKa and lipophilicity were modulated by a pending sulfonyl group, while good permeability and brain penetration were achieved via intramolecular hydrogen bonding. BACE1 selectivity over BACE2 was achieved in the S3 pocket by a novel bicyclic ring system. An optimization addressing reactive metabolite formation, cardiovascular safety, and CNS toxicity is described, leading to the clinical candidate JNJ-67569762 (12), which gave robust dose-dependent BACE1-mediated amyloid β lowering without showing BACE2-dependent hair depigmentation in preclinical models. We show that 12 has a favorable projected human dose and PK and hence presented us with an opportunity to test a highly selective BACE1 inhibitor in humans. However, 12 was found to have a QT effect upon repeat dosing in dogs and its development was halted in favor of other selective leads, which will be reported in the future.
中文翻译:

JNJ-67569762,一种靶向 S3 口袋的基于 2-氨基四氢吡啶的选择性 BACE1 抑制剂:从发现到临床候选
描述了一种新型 2-氨基四氢吡啶类 BACE1 抑制剂的发现。它们的pK一个和亲脂性,通过一个未决磺酰基调制,而良好的渗透性和脑渗透物经由分子内氢键来实现。BACE1 对 BACE2 的选择性是通过新型双环系统在 S3 口袋中实现的。描述了一种解决反应性代谢物形成、心血管安全性和 CNS 毒性的优化,导致临床候选 JNJ-67569762 ( 12 ),它提供了强大的剂量依赖性 BACE1 介导的β淀粉样蛋白降低,而在临床前模型中不显示 BACE2 依赖性毛发脱色. 我们证明12具有良好的预计人体剂量和 PK,因此为我们提供了在人体中测试高度选择性 BACE1 抑制剂的机会。然而,发现12在狗中重复给药时具有 QT 效应,并且其发展被停止,有利于其他选择性先导,这将在未来报告。
更新日期:2021-10-14
中文翻译:

JNJ-67569762,一种靶向 S3 口袋的基于 2-氨基四氢吡啶的选择性 BACE1 抑制剂:从发现到临床候选
描述了一种新型 2-氨基四氢吡啶类 BACE1 抑制剂的发现。它们的pK一个和亲脂性,通过一个未决磺酰基调制,而良好的渗透性和脑渗透物经由分子内氢键来实现。BACE1 对 BACE2 的选择性是通过新型双环系统在 S3 口袋中实现的。描述了一种解决反应性代谢物形成、心血管安全性和 CNS 毒性的优化,导致临床候选 JNJ-67569762 ( 12 ),它提供了强大的剂量依赖性 BACE1 介导的β淀粉样蛋白降低,而在临床前模型中不显示 BACE2 依赖性毛发脱色. 我们证明12具有良好的预计人体剂量和 PK,因此为我们提供了在人体中测试高度选择性 BACE1 抑制剂的机会。然而,发现12在狗中重复给药时具有 QT 效应,并且其发展被停止,有利于其他选择性先导,这将在未来报告。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号