当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Importance of Two-Electron Processes in Fe-Catalyzed Aryl-(hetero)aryl Cross-Couplings: Evidence of Fe0/FeII Couple Implication
Organometallics ( IF 2.5 ) Pub Date : 2021-09-22 , DOI: 10.1021/acs.organomet.1c00338
Vincent Wowk 1 , Lidie Rousseau 1, 2 , Guillaume Lefèvre 1
Organometallics ( IF 2.5 ) Pub Date : 2021-09-22 , DOI: 10.1021/acs.organomet.1c00338
Vincent Wowk 1 , Lidie Rousseau 1, 2 , Guillaume Lefèvre 1
Affiliation
|
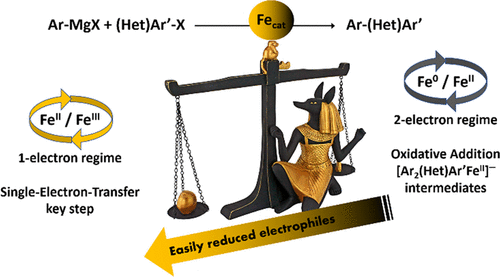
We demonstrate in this work that two drastically distinct mechanisms can be involved in aryl-(hetero)aryl Fe-mediated cross-couplings between Grignard reagents and organic halides, depending on the nature of the latter. (Hetero)aryl electrophiles, which easily undergo one-electron reduction, can be involved in a FeII/FeIII coupling sequence featuring an in situ generated organoiron(II) species, akin to their aliphatic analogues. On the other hand, less easily reduced substrates can be activated by transient Fe0 species formed by the reduction of the precatalyst. In this case, the coupling mechanism relies on two-electron elementary steps involving the Fe0/FeII redox couple and proceeds by an oxidative addition/reductive elimination sequence. Hammett analysis shows that both those elementary steps are faster for electrophiles substituted by electron-withdrawing groups. The two mechanisms discussed herein can be involved concomitantly for electrophiles displaying an average oxidative power. Attesting to the feasibility of the aforementioned bielectronic mechanism, high-spin organoiron(II) intermediates formed by two-electron oxidative addition onto (hetero)aryl halides in catalytically relevant conditions were also characterized for the first time. Those results are sustained by paramagnetic 1H NMR, kinetics monitoring, and density functional theory (DFT) calculations.
中文翻译:

双电子过程在 Fe 催化的芳基-(杂)芳基交叉偶联中的重要性:Fe0/FeII 耦合暗示的证据
我们在这项工作中证明了两种截然不同的机制可能涉及格氏试剂和有机卤化物之间的芳基-(杂)芳基 Fe 介导的交叉偶联,具体取决于后者的性质。(杂)芳基亲电试剂很容易进行单电子还原,可以参与 Fe II / Fe III偶联序列,其特征是原位生成的有机铁 (II) 物质,类似于它们的脂肪族类似物。另一方面,不易被还原的底物可以被由预催化剂还原形成的瞬态 Fe 0物质激活。在这种情况下,耦合机制依赖于涉及 Fe 0 /Fe II 的双电子基本步骤氧化还原对并通过氧化加成/还原消除顺序进行。Hammett 分析表明,对于被吸电子基团取代的亲电试剂,这两个基本步骤都更快。对于显示平均氧化能力的亲电试剂,本文讨论的两种机制可以同时涉及。证明上述双电子机制的可行性,还首次表征了在催化相关条件下通过双电子氧化加成到(杂)芳基卤化物上形成的高自旋有机铁(II)中间体。这些结果得到了顺磁1 H NMR、动力学监测和密度泛函理论 (DFT) 计算的支持。
更新日期:2021-10-11
中文翻译:

双电子过程在 Fe 催化的芳基-(杂)芳基交叉偶联中的重要性:Fe0/FeII 耦合暗示的证据
我们在这项工作中证明了两种截然不同的机制可能涉及格氏试剂和有机卤化物之间的芳基-(杂)芳基 Fe 介导的交叉偶联,具体取决于后者的性质。(杂)芳基亲电试剂很容易进行单电子还原,可以参与 Fe II / Fe III偶联序列,其特征是原位生成的有机铁 (II) 物质,类似于它们的脂肪族类似物。另一方面,不易被还原的底物可以被由预催化剂还原形成的瞬态 Fe 0物质激活。在这种情况下,耦合机制依赖于涉及 Fe 0 /Fe II 的双电子基本步骤氧化还原对并通过氧化加成/还原消除顺序进行。Hammett 分析表明,对于被吸电子基团取代的亲电试剂,这两个基本步骤都更快。对于显示平均氧化能力的亲电试剂,本文讨论的两种机制可以同时涉及。证明上述双电子机制的可行性,还首次表征了在催化相关条件下通过双电子氧化加成到(杂)芳基卤化物上形成的高自旋有机铁(II)中间体。这些结果得到了顺磁1 H NMR、动力学监测和密度泛函理论 (DFT) 计算的支持。







































 京公网安备 11010802027423号
京公网安备 11010802027423号