Journal of Photochemistry and Photobiology A: Chemistry ( IF 4.1 ) Pub Date : 2021-09-20 , DOI: 10.1016/j.jphotochem.2021.113557 Brij Mohan 1 , Krunal Modi 2 , Jaymin Parikh 2 , Shixuan Ma 1 , Sandeep Kumar 1 , Krishna Kumar Manar 1 , Feiyun Sun 3 , Hengzhi You 1 , Peng Ren 1
|
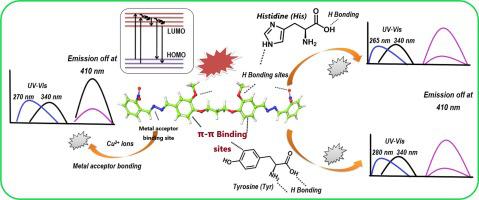
Spectroscopic detection of metal ions and amino acids has gained significant attention owing to the public health domain. In this study, we design and synthesize a rapid UV–visible and fluorescence sensor 1,3-bis(2-methoxy-4-((E)-(((E)-2-nitrobenzylidene)hydrazineylidene)methyl)phenoxy)propane (BMNHMPP) for the selective detection of Cu2+ ions (limit of detection (LOD) ≈ 14–18 × 10−7 M, linear range = 0–130 μM, and the binding constant KCu = 3.30 × 1010 M−2) through turn-off response. The reversibility and snatching of BMNHMPP-Cu2+ complex with EDTA, cysteine (Cys), proline (Pro), and threonine (Thr) in a solvent systemis demonstrated with the restoring of emission intensity. Moreover, the BMNHMPP shows the blue shift in ultraviolet UV–visible and turn-off selective response for the detection of Histidine (His) (LOD ≈ 6–11 × 10−7 M, KHis = 6.53 × 104 M−1 and range of 0–80 μM) and Tyrosine (Tyr) (LOD ≈ 8–13 × 10−7 M, and KTyr = 2.13 × 1010 M−2, range of 0–112 μM). Also, the binding behavior of BMNHMPP with Cu2+, His and Tyr, quantum yields, binding stoichiometry and pH effects are determined using spectroscopic techniques. The mechanistic insights of metal acceptor bonds (BMNHMPP-Cu2+), H-bonding (BMNHMPP-His), H-bonding, and π–π edge to face bondings (BMNHMPP-Tyr-Tyr) were studied. The in-silico study provides a better understanding of the structural behavior and binding mode with the binding and docking energy values. Moreover, the time-dependent density functional theory calculation provides a proper highlight of the frontier molecular orbitals (FMOs).
中文翻译:

基于 2-硝基亚苄基肼的选择性快速传感器检测 Cu2+ 离子、组氨酸和酪氨酸的功效:光谱和计算研究
由于公共卫生领域,金属离子和氨基酸的光谱检测受到了极大的关注。在本研究中,我们设计并合成了一种快速紫外可见荧光传感器 1,3-双(2-甲氧基-4-((E)-(((E)-2-硝基亚苄基)肼基亚甲基)甲基)苯氧基)丙烷( BMNHMPP ) 用于选择性检测 Cu 2+离子(检测限 (LOD) ≈ 14–18 × 10 -7 M,线性范围 = 0–130 μM,结合常数 K Cu = 3.30 × 10 10 M - 2)通过关断响应。BMNHMPP-Cu 2 +的可逆性和抢夺性与 EDTA、半胱氨酸 (Cys)、脯氨酸 (Pro) 和苏氨酸 (Thr) 在溶剂系统中的复合物证明了发射强度的恢复。此外,BMNHMPP显示紫外-可见光和关闭选择性响应的蓝移,用于检测组氨酸(His)(LOD ≈ 6–11 × 10 -7 M,K His = 6.53 × 10 4 M -1和0–80 μM) 和酪氨酸 (Tyr)(LOD ≈ 8–13 × 10 -7 M,K Tyr = 2.13 × 10 10 M -2,范围 0–112 μM)。此外,BMNHMPP与 Cu 2+的结合行为、His 和 Tyr、量子产率、结合化学计量和 pH 值效应是使用光谱技术确定的。研究了金属受体键 ( BMNHMPP -Cu 2+ )、氢键 ( BMNHMPP -His)、氢键和 π-π 边对面键 ( BMNHMPP -Tyr-Tyr) 的机理。计算机内研究通过结合和对接能量值更好地了解结构行为和结合模式。此外,瞬态密度泛函理论计算提供了前沿分子轨道 (FMO) 的适当亮点。































 京公网安备 11010802027423号
京公网安备 11010802027423号