Journal of Molecular Structure ( IF 4.0 ) Pub Date : 2021-09-21 , DOI: 10.1016/j.molstruc.2021.131544 Lorena Camargo-Ayala 1 , Luis Prent-Peñaloza 2 , Efraín Polo-Cuadrado 3 , Iván Brito 4 , Jonathan Cisterna 4 , Edison Osorio 5 , Wendy González 6 , Margarita Gutiérrez 3
|
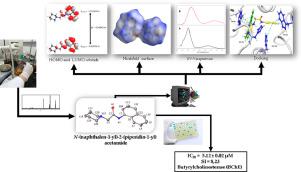
In this study, we reported for the first time the crystalline and molecular structure of the compound N-(naphthalen-1-yl) -2- (piperidin-1-yl) acetamide. The synthesis of the compound was carried out by amidation using a coupling reagent (N, N'-diisopropylcarbodiimide) and subsequently amination. The compound was experimentally characterized by UV-visible spectroscopy, 13C-NMR, 1H-NMR, melting point, and X-ray diffraction technique. In addition, we include here, theoretical studies on boundary molecular orbitals, descriptors global reactivity, natural bond orbital analysis, Hirshfeld surface analysis, energy framework and molecular docking. Interestingly, acetamide shows a keto form in the solid state, as reported in similar monosubstituted acetamide compounds. The dihedral angles are negligible, being essentially coplanar between the fragment. Additionally, inhibitory activity studies were carried out for the enzymes acetylcholinesterase and butyrylcholinesterase, obtaining an IC50 of 426.14 ± 18.54 µM and 5.12 ± 0.02 µM respectively, thus having significant selectivity for butyrylcholinesterase with an IC50 lower than that reported for the reference compound galantamine (7.96 ± 0.8 µM). Our consistent molecular coupling analyzes show the formation of a more stable complex between compound 5 and butyricholinesterase due to the complementary interactions of compound 5′s naphthyl ring with residues Trp 231 and Phe 329 compared to the complex formed with acetylcholinesterase.
中文翻译:

选择性丁酰胆碱酯酶抑制剂 N-(naphthalen-1-yl)-2-(piperidin-1-yl) 乙酰胺的合成、表征、晶体和分子结构及理论研究
在这项研究中,我们首次报道了化合物N- (naphthalen-1-yl) -2- (piperidin-1-yl) 乙酰胺的晶体和分子结构。化合物的合成通过使用偶联剂( N,N'-二异丙基碳二亚胺)的酰胺化和随后的胺化来进行。该化合物通过紫外-可见光谱、13 C-NMR、1H-NMR、熔点和 X 射线衍射技术。此外,我们在这里还包括边界分子轨道的理论研究、全局反应性描述符、自然键轨道分析、Hirshfeld 表面分析、能量框架和分子对接。有趣的是,乙酰胺在固态下显示出酮形式,正如在类似的单取代乙酰胺化合物中所报道的那样。二面角可以忽略不计,碎片之间基本上是共面的。此外,对乙酰胆碱酯酶和丁酰胆碱酯酶进行了抑制活性研究,获得的 IC 50 分别为 426.14 ± 18.54 µM 和 5.12 ± 0.02 µM,因此对丁酰胆碱酯酶具有显着的选择性,IC 50低于参考化合物加兰他敏 (7.96 ± 0.8 µM) 的报告值。我们一致的分子偶联分析表明,与乙酰胆碱酯酶形成的复合物相比,由于化合物 5 的萘基环与残基 Trp 231 和 Phe 329 的互补相互作用,在化合物5和丁酰胆碱酯酶之间形成了更稳定的复合物。































 京公网安备 11010802027423号
京公网安备 11010802027423号