当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism of Alkyl Chloroformate-Mediated Esterification of Carboxylic Acids in Aqueous Media
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-09-21 , DOI: 10.1021/acs.joc.1c01546
Stanislav Opekar 1 , Jaroslav Kvíčala 2 , Martin Moos 1 , Vladimír Pejchal 3 , Petr Šimek 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-09-21 , DOI: 10.1021/acs.joc.1c01546
Stanislav Opekar 1 , Jaroslav Kvíčala 2 , Martin Moos 1 , Vladimír Pejchal 3 , Petr Šimek 1
Affiliation
|
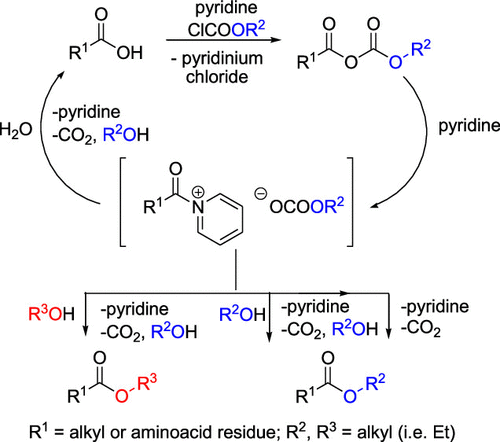
The protection of carboxyl groups by esterification has been the most common method in macroscale and microscale chemistries. The esterification is usually conducted under anhydrous conditions; however, in biological chemistry and related fields, the reaction is of major concern in aqueous environments. Immediate esterification of the carboxyl in aqueous alcoholic media driven by an alkyl chloroformate and pyridine has been such a method which has found widespread use in many research and industrial laboratories. Nevertheless, the reaction mechanism has not yet been investigated, to our knowledge, and is not well understood. Herein, we describe the reaction intermediates and demonstrate that the reaction proceeds via a continual formation of the N-acylpyridinium intermediate decomposed by several reaction channels to the final ester. The understanding of the mechanism could encourage novel laboratory applications of this important esterification method.
中文翻译:

水介质中氯甲酸烷基酯介导的羧酸酯化机理
通过酯化保护羧基是宏观和微观化学中最常用的方法。酯化反应通常在无水条件下进行;然而,在生物化学和相关领域,该反应是水环境中的主要关注点。由氯甲酸烷基酯和吡啶驱动的水性醇介质中的羧基立即酯化是一种已在许多研究和工业实验室中广泛使用的方法。然而,据我们所知,反应机理尚未被研究,也不是很清楚。在这里,我们描述了反应中间体,并证明反应是通过N的连续形成进行的。-酰基吡啶鎓中间体通过几个反应通道分解成最终酯。对该机制的理解可以鼓励这种重要的酯化方法在实验室中的新应用。
更新日期:2021-09-21
中文翻译:

水介质中氯甲酸烷基酯介导的羧酸酯化机理
通过酯化保护羧基是宏观和微观化学中最常用的方法。酯化反应通常在无水条件下进行;然而,在生物化学和相关领域,该反应是水环境中的主要关注点。由氯甲酸烷基酯和吡啶驱动的水性醇介质中的羧基立即酯化是一种已在许多研究和工业实验室中广泛使用的方法。然而,据我们所知,反应机理尚未被研究,也不是很清楚。在这里,我们描述了反应中间体,并证明反应是通过N的连续形成进行的。-酰基吡啶鎓中间体通过几个反应通道分解成最终酯。对该机制的理解可以鼓励这种重要的酯化方法在实验室中的新应用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号