当前位置:
X-MOL 学术
›
Macromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Functionalization Methodology for Synthesis of Silane-End-Functionalized Linear and Star Poly(aryl isocyanide)s by Combination of Cationic Polymerization and Hydrosilylation Reaction
Macromolecules ( IF 5.1 ) Pub Date : 2021-09-20 , DOI: 10.1021/acs.macromol.1c01001 Tuemay Abadi Belay 1 , Jupeng Chen 1 , Huan Xu 1 , Shaowen Zhang 1 , Shilu Chen 1 , Xiaofang Li 1
Macromolecules ( IF 5.1 ) Pub Date : 2021-09-20 , DOI: 10.1021/acs.macromol.1c01001 Tuemay Abadi Belay 1 , Jupeng Chen 1 , Huan Xu 1 , Shaowen Zhang 1 , Shilu Chen 1 , Xiaofang Li 1
Affiliation
|
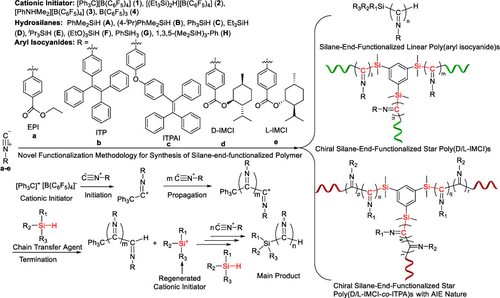
The combination of metal-free borates or borane mediated nonliving cationic polymerization of aryl isocyanides and hydrosilylation reaction of hydrosilanes affords a general and effective functionalization methodology to synthesize silane-end-functionalized linear and star poly(aryl isocyanide)s. The configurations, end group types, and molecular weights of these silane-end-functionalized poly(aryl isocyanide)s can be easily tuned by varying the type and concentration of hydrosilanes. A variety of monofunctional hydrosilanes such as PhMe2SiH, (4-iPrC6H4)Me2SiH, Ph3SiH, Et3SiH, iPr3SiH, and (OEt)3SiH have been successfully applied to the synthesis of linear poly(aryl isocyanide)s with different silane-end-functional groups having a one-handed helical conformation or an aggregation-induced emission (AIE) characteristic (activity up to 1.5 × 107 g/(mol of cat.·h), Mn up to 8.4 × 105), while two multifunctional hydrosilanes PhSiH3 and 1,3,5-(Me2SiH)3C6H3 are conductive to prepare star poly(aryl isocyanide)s and copoly(aryl isocyanide)s having one central core of multifunctional silane with a one-handed helical conformation and/or an AIE characteristic (activity up to 3.4 × 106 g/(mol of cat.·h), Mn up to 9.1 × 104). 1H, 29Si, and 1H-29Si HMBC NMR, Fourier transform infrared spectroscopy (FT-IR), electrospray-ionization mass spectrometry (ESI-MS), and matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF-MS) analysis confirm the presence of silane-end-functional groups in these linear and star poly(aryl isocyanide)s. The mechanism study based on density functional theory (DFT) calculations suggests that hydrosilane plays a dual functional role of the chain transfer agent and regenerated cationic initiator in the [Ph3C][B(C6F5)4]-initiated cationic polymerization of aryl isocyanides.
中文翻译:

阳离子聚合和氢化硅烷化反应合成硅烷末端官能化线性和星形聚(芳基异氰化物)的官能化方法
无金属硼酸盐或硼烷介导的芳基异氰化物非活性阳离子聚合和氢硅烷的氢化硅烷化反应的组合提供了一种通用且有效的官能化方法来合成硅烷末端官能化的线性和星形聚(芳基异氰化物)。通过改变氢硅烷的类型和浓度,可以轻松调整这些硅烷端基官能化聚(芳基异氰)的构型、端基类型和分子量。各种单官能氢硅烷,例如 PhMe 2 SiH、(4- i PrC 6 H 4 )Me 2 SiH、Ph 3 SiH、Et 3 SiH、i Pr 3 SiH 和 (OEt)3 SiH 已成功应用于合成具有单手螺旋构象或聚集诱导发射 (AIE) 特性的不同硅烷末端官能团的线性聚(芳基异氰化物)(活性高达 1.5 × 10 7克/(猫摩尔。·h)时,中号ñ高达8.4×10 5),而2多功能含氢硅烷PhSiH 3和1,3,5-(ME 2 SiH)的3 ç 6 ħ 3是导电的,以制备聚星(芳基异氰化物)和共聚(芳基异氰化物)具有一个具有单手螺旋构象和/或 AIE 特征的多功能硅烷中心核(活性高达 3.4 × 10 6g/(mol of cat.·h),M n高达 9.1 × 10 4 )。1 H、29 Si 和1 H- 29 Si HMBC NMR、傅里叶变换红外光谱 (FT-IR)、电喷雾电离质谱 (ESI-MS) 和基质辅助激光解吸电离飞行时间质谱 ( MALDI-TOF-MS) 分析证实在这些线性和星形聚(芳基异氰化物)中存在硅烷末端官能团。基于密度泛函理论 (DFT) 计算的机理研究表明,氢硅烷在 [Ph 3 C][B(C 6 F 5 ) 中起到链转移剂和再生阳离子引发剂的双重功能作用4 ]-引发的芳基异氰化物的阳离子聚合。
更新日期:2021-10-12
中文翻译:

阳离子聚合和氢化硅烷化反应合成硅烷末端官能化线性和星形聚(芳基异氰化物)的官能化方法
无金属硼酸盐或硼烷介导的芳基异氰化物非活性阳离子聚合和氢硅烷的氢化硅烷化反应的组合提供了一种通用且有效的官能化方法来合成硅烷末端官能化的线性和星形聚(芳基异氰化物)。通过改变氢硅烷的类型和浓度,可以轻松调整这些硅烷端基官能化聚(芳基异氰)的构型、端基类型和分子量。各种单官能氢硅烷,例如 PhMe 2 SiH、(4- i PrC 6 H 4 )Me 2 SiH、Ph 3 SiH、Et 3 SiH、i Pr 3 SiH 和 (OEt)3 SiH 已成功应用于合成具有单手螺旋构象或聚集诱导发射 (AIE) 特性的不同硅烷末端官能团的线性聚(芳基异氰化物)(活性高达 1.5 × 10 7克/(猫摩尔。·h)时,中号ñ高达8.4×10 5),而2多功能含氢硅烷PhSiH 3和1,3,5-(ME 2 SiH)的3 ç 6 ħ 3是导电的,以制备聚星(芳基异氰化物)和共聚(芳基异氰化物)具有一个具有单手螺旋构象和/或 AIE 特征的多功能硅烷中心核(活性高达 3.4 × 10 6g/(mol of cat.·h),M n高达 9.1 × 10 4 )。1 H、29 Si 和1 H- 29 Si HMBC NMR、傅里叶变换红外光谱 (FT-IR)、电喷雾电离质谱 (ESI-MS) 和基质辅助激光解吸电离飞行时间质谱 ( MALDI-TOF-MS) 分析证实在这些线性和星形聚(芳基异氰化物)中存在硅烷末端官能团。基于密度泛函理论 (DFT) 计算的机理研究表明,氢硅烷在 [Ph 3 C][B(C 6 F 5 ) 中起到链转移剂和再生阳离子引发剂的双重功能作用4 ]-引发的芳基异氰化物的阳离子聚合。

































 京公网安备 11010802027423号
京公网安备 11010802027423号