European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2021-09-20 , DOI: 10.1016/j.ejmech.2021.113860 Surendra Kunwar 1 , Soo-Yeon Hwang 2 , Pramila Katila 1 , Seojeong Park 2 , Kyung-Hwa Jeon 2 , Daeun Kim 2 , Tara Man Kadayat 1 , Youngjoo Kwon 2 , Eung-Seok Lee 1
|
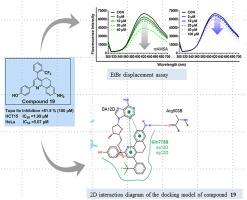
Several anticancer agents have been developed and innovative approaches have been made toward cancer type-specific medicines for cancer treatment. As a continuous effort to develop potential chemotherapeutic agents, a novel series of 2,4-diphenyl-5,6-dihydrobenzo(h)quinolin-8-amines containing amino groups, hydroxyphenyl and fluorine functionalities were designed and synthesized. The compounds were evaluated for topo IIα inhibitory and cytotoxicity against HCT15, and HeLa human cancer cell lines. Among synthesized thirty compounds, the majority exhibited strong topo IIα inhibition and anti-proliferation against HCT15 colorectal adenocarcinoma cell line. The structure-activity relationship study revealed that compounds with –CF3 and –OCF3 substituents at 4- position and 3′ or 4′-hydroxyphenyl at 2-position attached to the central pyridine ring displayed potent topo IIα and anti-proliferative activity in colorectal and cervix cancer cell line. In vitro studies provided the evidence that compounds 16, 19, 22, and 28 possess excellent topo IIα inhibition and antiproliferative activity. For a better understanding, topo IIα cleavage complex, EtBr displacement, KI quenching assays and molecular docking of compound 19 was performed and the results revealed the mode of action as a DNA intercalative topo IIα poison inhibitor. The results obtained from this study provide insight into the DNA binding mechanism of 2,4-diphenyl-5,6-dihydrobenzo(h)quinolin-8-amines and alteration in topo IIα inhibitory and antiproliferative activity with modifications in the rigid structure.
中文翻译:

发现 2,4-二苯基-5,6-二氢苯并(h)喹啉-8-胺衍生物作为新型 DNA 插入拓扑异构酶 IIα 毒物
已经开发了几种抗癌剂,并且已经针对用于癌症治疗的癌症类型特异性药物提出了创新方法。作为开发潜在化疗药物的持续努力,设计和合成了一系列含有氨基、羟苯基和氟官能团的新型 2,4-二苯基-5,6-二氢苯并(h)喹啉-8-胺。评估了这些化合物对 HCT15 和 HeLa 人类癌细胞系的拓扑 IIα 抑制和细胞毒性。在合成的 30 种化合物中,大多数化合物对 HCT15 结直肠腺癌细胞系表现出强烈的 topo IIα 抑制和抗增殖作用。构效关系研究表明,具有–CF 3和–OCF 3的化合物与中央吡啶环相连的 4 位取代基和 2 位 3' 或 4'-羟基苯基在结肠直肠癌细胞系和宫颈癌细胞系中显示出有效的 topo IIα 和抗增殖活性。体外研究提供了化合物16、19、22和28具有优异的拓扑 IIα 抑制和抗增殖活性的证据。为了更好地理解,topo IIα 裂解复合物、EtBr 置换、KI 猝灭测定和化合物19的分子对接进行了,结果揭示了作为 DNA 插入拓扑 IIα 毒物抑制剂的作用方式。从这项研究中获得的结果提供了对 2,4-diphenyl-5,6-dihydrobenzo(h)quinolin-8-amines 的 DNA 结合机制的深入了解,以及通过改变刚性结构来改变 topo IIα 抑制和抗增殖活性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号