当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
One-pot synthesis of 2-methylfurans from 3-(trimethylsilyl)propargyl acetates promoted by trimethylsilyl trifluoromethanesulfonate
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2021-09-20 , DOI: 10.1016/j.tetlet.2021.153424 Danielle E. Sklar 1 , Alex V. Helbling 1 , Yiqi Liu 1 , C. Wade Downey 1
中文翻译:

在三氟甲磺酸三甲基甲硅烷基酯的促进下,由 3-(三甲基甲硅烷基)炔丙酯乙酸酯一锅法合成 2-甲基呋喃
更新日期:2021-09-20
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2021-09-20 , DOI: 10.1016/j.tetlet.2021.153424 Danielle E. Sklar 1 , Alex V. Helbling 1 , Yiqi Liu 1 , C. Wade Downey 1
Affiliation
|
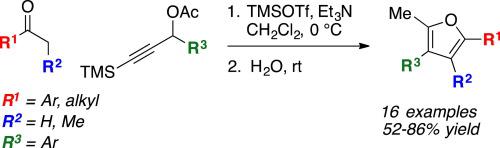
In the presence of trimethylsilyl trifluoromethanesulfonate (TMSOTf) and triethylamine, 3-(trimethylsilyl)propargyl carboxylates undergo a one-pot alkylation-cyclization-desilylation reaction with ketones to produce 2-methylfurans. Alkylation at 0 °C in methylene chloride, followed by acid-catalyzed cyclization at room temperature, provides the furans in 52–86% yield. Cyclization and desilylation appear to be promoted by triflic acid generated in situ from the exposure of the reaction mixture to water upon completion of the initial substitution reaction.
中文翻译:

在三氟甲磺酸三甲基甲硅烷基酯的促进下,由 3-(三甲基甲硅烷基)炔丙酯乙酸酯一锅法合成 2-甲基呋喃
在三氟甲磺酸三甲基甲硅烷基酯 (TMSOTf) 和三乙胺的存在下,羧酸 3-(三甲基甲硅烷基)炔丙基酯与酮进行一锅烷基化-环化-脱甲硅烷基化反应,生成 2-甲基呋喃。在 0 °C 下在二氯甲烷中烷基化,然后在室温下酸催化环化,以 52-86% 的产率提供呋喃。环化和脱甲硅烷基化似乎由在初始取代反应完成后将反应混合物暴露于水中而原位生成的三氟甲磺酸促进。















































 京公网安备 11010802027423号
京公网安备 11010802027423号