Journal of Industrial and Engineering Chemistry ( IF 5.9 ) Pub Date : 2021-09-20 , DOI: 10.1016/j.jiec.2021.09.015
Rubieli Carla Frezza Zeferino 1, 2 , Vinicius Aleixo Angonese Piaia 2 , Vinícius Tres Orso 2 , Vítor Machado Pinheiro 2 , Micheli Zanetti 2, 3 , Gustavo Lopes Colpani 2, 3 , Natan Padoin 1 , Cíntia Soares 1 , Márcio Antônio Fiori 1, 2, 3 , Humberto Gracher Riella 1
|
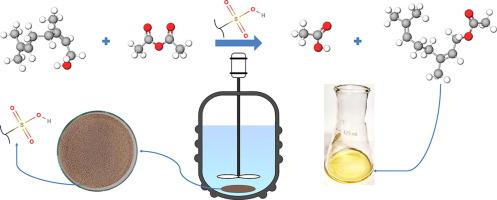
This work aims to synthesize neryl acetate from the nerol esterification reaction with acetic anhydride through heterogeneous catalysis using the ion exchange resin Lewatit® GF 101. The reaction was monitored by gas chromatography and the neryl acetate chemical structure was confirmed by mass spectrometry and nuclear magnetic resonance spectroscopy. The variables effect on the neryl acetate synthesis was evaluated from an experimental design analysis. The reaction showed the highest combined values for nerol conversion (98.11%) and neryl acetate selectivity (86.10%) at 30 min within optimal experimental conditions of temperature at 40 °C, catalyst content at 7% wt, molar ratio at 1:4 (nerol: acetic anhydride), agitation speed at 250 rpm and nerol content at 3 mmol. The complete nerol conversion was achieved at 40 min with 82.34% selectivity. The reaction rate was controlled only by the nerol decay, an expected behavior due to the excess of acetic anhydride used. In addition, the value obtained for the main reaction kinetic constant found by a pseudo-homogeneous model was 6 times greater than that of the parallel reaction. The catalyst reuse was investigated and after 3 cycles a high conversion (96.68%) and selectivity (83.78%) were observed indicating a low loss of the catalytic activity.
中文翻译:

离子交换树脂非均相催化橙花醇与醋酸酐酯化合成醋酸橙花酯
本工作旨在使用离子交换树脂 Lewatit® GF 101 通过多相催化与醋酸酐通过橙花醇酯化反应合成醋酸橙花酯。该反应通过气相色谱进行监测,醋酸橙花酯的化学结构通过质谱和核磁共振进行确认光谱学。从实验设计分析评估了对醋酸橙花酯合成的变量影响。在温度为 40 °C、催化剂含量为 7% wt、摩尔比为 1:4 的最佳实验条件下,该反应在 30 分钟时表现出最高的橙花醇转化率 (98.11%) 和醋酸橙花酯选择性 (86.10%) 的综合值(橙花醇:乙酸酐),搅拌速度为 250 rpm,橙花醇含量为 3 mmol。橙花醇在 40 分钟时完全转化,选择性为 82.34%。反应速率仅由橙花醇衰变控制,这是由于使用过量乙酸酐而导致的预期行为。此外,由拟均相模型求得的主反应动力学常数值是平行反应的 6 倍。对催化剂再利用进行了研究,并且在 3 个循环后观察到高转化率 (96.68%) 和选择性 (83.78%),表明催化活性的损失很小。

































 京公网安备 11010802027423号
京公网安备 11010802027423号