当前位置:
X-MOL 学术
›
Biopharm. Drug Dispos.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Comparison of tissue pharmacokinetics of esflurbiprofen plaster with flurbiprofen tablets in patients with knee osteoarthritis: A multicenter randomized controlled trial
Biopharmaceutics & Drug Disposition ( IF 1.7 ) Pub Date : 2021-09-16 , DOI: 10.1002/bdd.2302 Masaki Amemiya 1 , Yusuke Nakagawa 1 , Hideya Yoshimura 2 , Toru Takahashi 2 , Kei Inomata 3 , Tsuyoshi Nagase 3 , Young-Jin Ju 4 , Masayuki Shimaya 5 , Sachiyuki Tsukada 6 , Naoyuki Hirasawa 6 , Hideyuki Koga 1
Biopharmaceutics & Drug Disposition ( IF 1.7 ) Pub Date : 2021-09-16 , DOI: 10.1002/bdd.2302 Masaki Amemiya 1 , Yusuke Nakagawa 1 , Hideya Yoshimura 2 , Toru Takahashi 2 , Kei Inomata 3 , Tsuyoshi Nagase 3 , Young-Jin Ju 4 , Masayuki Shimaya 5 , Sachiyuki Tsukada 6 , Naoyuki Hirasawa 6 , Hideyuki Koga 1
Affiliation
|
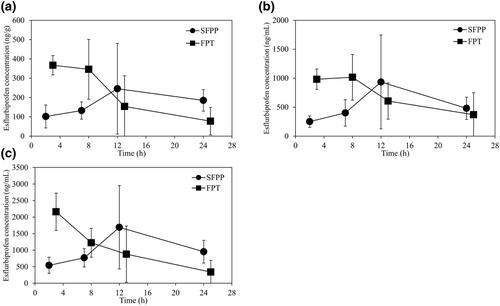
This open-label, multicenter, prospective, randomized controlled trial aimed to determine the effectiveness of esflurbiprofen plaster (SFPP) and flurbiprofen tablets (FPTs) on knee osteoarthritis in patients scheduled for total knee arthroplasty by comparing the transfer of esflurbiprofen and flurbiprofen to tissues and fluids. Thirty-eight patients were randomly assigned in a 1:1 ratio to receive SFPP or FPT. Both groups were then divided into four subgroups, according to whether they received the final dose of SFPP or FPT at 2, 7, 12, or 24 h before planned surgery. The primary endpoints were the esflurbiprofen concentrations in synovium, synovial fluid, and plasma. Areas under concentration–time curves (AUC0–24 h) of esflurbiprofen were calculated for each group. Pain was assessed using a numeric rating scale (NRS) 7 days before and immediately before surgery. The AUC0–24 h in the synovium were 4401.24 and 4862.70 ng·h/g in the SFPP and FPT groups, respectively. Maximum esflurbiprofen concentrations were observed in the synovium, synovial fluids, and plasma after SFPP application for 12 h. The NRS results indicated a long-lasting effect of SFPP. The AUC of the synovial esflurbiprofen concentration of SFPP indicated that the SFPP is transferred to the synovium and synovial fluid in high concentration. The efficient deep-tissue transfer of esflurbiprofen suggests that its pharmacokinetic characteristics differ from those of conventional topical NSAIDs. This study was prospectively registered in the Japan Registry of Clinical Trials (registration number: jRCTs031180228).
中文翻译:

依氟比洛芬膏与氟比洛芬片在膝关节骨性关节炎患者中的组织药代动力学比较:一项多中心随机对照试验
这项开放标签、多中心、前瞻性、随机对照试验旨在通过比较艾氟比洛芬和氟比洛芬向组织的转移和流体。38 名患者以 1:1 的比例随机分配接受 SFPP 或 FPT。然后根据他们是否在计划手术前 2、7、12 或 24 小时接受最终剂量的 SFPP 或 FPT 将两组分为四个亚组。主要终点是滑膜、滑液和血浆中的依氟比洛芬浓度。浓度-时间曲线下的面积(AUC 0-24 h) 计算每组的esflurbiprofen。在手术前 7 天和手术前立即使用数字评分量表 (NRS) 评估疼痛。AUC 0-24 小时SFPP 组和 FPT 组滑膜中分别为 4401.24 和 4862.70 ng·h/g。在 SFPP 应用 12 小时后,在滑膜、滑液和血浆中观察到最大的依氟比洛芬浓度。NRS 结果表明 SFPP 的持久效果。SFPP的依氟比洛芬滑膜浓度的AUC表明SFPP以高浓度转移到滑膜和滑液中。依氟比洛芬的有效深层组织转移表明其药代动力学特征不同于传统的局部非甾体抗炎药。本研究前瞻性地在日本临床试验注册中心注册(注册号:jRCTs031180228)。
更新日期:2021-09-16
中文翻译:

依氟比洛芬膏与氟比洛芬片在膝关节骨性关节炎患者中的组织药代动力学比较:一项多中心随机对照试验
这项开放标签、多中心、前瞻性、随机对照试验旨在通过比较艾氟比洛芬和氟比洛芬向组织的转移和流体。38 名患者以 1:1 的比例随机分配接受 SFPP 或 FPT。然后根据他们是否在计划手术前 2、7、12 或 24 小时接受最终剂量的 SFPP 或 FPT 将两组分为四个亚组。主要终点是滑膜、滑液和血浆中的依氟比洛芬浓度。浓度-时间曲线下的面积(AUC 0-24 h) 计算每组的esflurbiprofen。在手术前 7 天和手术前立即使用数字评分量表 (NRS) 评估疼痛。AUC 0-24 小时SFPP 组和 FPT 组滑膜中分别为 4401.24 和 4862.70 ng·h/g。在 SFPP 应用 12 小时后,在滑膜、滑液和血浆中观察到最大的依氟比洛芬浓度。NRS 结果表明 SFPP 的持久效果。SFPP的依氟比洛芬滑膜浓度的AUC表明SFPP以高浓度转移到滑膜和滑液中。依氟比洛芬的有效深层组织转移表明其药代动力学特征不同于传统的局部非甾体抗炎药。本研究前瞻性地在日本临床试验注册中心注册(注册号:jRCTs031180228)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号