Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2021-09-17 , DOI: 10.1016/j.molliq.2021.117549
Aysha Fatima 1, 2 , Meenakshi Singh 1 , Neha Agarwal 1 , Indresh Verma 3 , Ray J. Butcher 4 , Nazia Siddiqui 5 , Saleem Javed 1
|
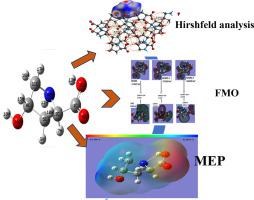
In present study, title compound Trans-4-Hydroxy-L-Proline is investigated theoretically and experimentally by FT-IR, NMR and UV–Vis spectra. The theoretical optimized geometrical parameters, vibrational analysis were performed by Density Functional Theory (DFT) with the B3LYP method at 6-311++G(d,p) basis set. Hirshfeld surface analysis has been performed and studied the nature of intermolecular interactions within the crystal structure via 3D and 2D surfaces, and demonstrated the H-bonding with close contacts in crystal via dnorm surface. Local chemical activity was studied via molecular electrostatic potential (MEP) and analyzed the reactive areas of the titled molecule, and local chemical reactivity was studied by population analysis and Fukui function analysis. FMOs investigation given various chemical activity parameters like- chemical hardness, chemical softness and electrophilicity index. NLO has been performed and analyzed the hyperpolarizability value and polarity of molecule. Natural Bond Orbital (NBO) analysis was carried out, reporting the hybridization of atoms that form bonds. The charge transfer of title molecule have been examined by TD-DFT method. The compound was docked with (A) 1TKZ, (B) 4WKE, (C) 5KK7, (D) 5OSC, (E) 6B6G, (F) 6HSN, and (G) 6YW0 Protein receptors to find the best ligand–protein interactions, the best and lowest binding energy obtained was −5.4 Kcal/mol. Drug likeness was also performed on titled molecule and its different derivatives for the confirmation of drug-like character of titled molecule.
中文翻译:

通过 DFT 方法对反式-4-羟基-L-脯氨酸进行光谱、分子结构、电子、Hirshfeld 表面、分子对接和热力学研究
在本研究中,标题化合物Trans-4-Hydroxy-L-Proline 通过 FT-IR、NMR 和 UV-Vis 光谱进行理论和实验研究。理论优化的几何参数、振动分析通过密度泛函理论 (DFT) 和 B3LYP 方法在 6-311++G(d,p) 基组下进行。Hirshfeld 表面分析已通过 3D 和 2D 表面进行并研究了晶体结构内分子间相互作用的性质,并通过 dnorm 表面证明了与晶体中紧密接触的 H 键合。通过分子静电势(MEP)研究局部化学活性并分析标题分子的反应面积,并通过群体分析和Fukui函数分析研究局部化学反应。FMOs 调查给出了各种化学活性参数,如化学硬度、化学柔软度和亲电性指数。已进行 NLO 并分析了分子的超极化值和极性。进行了自然键轨道 (NBO) 分析,报告了形成键的原子的杂化。标题分子的电荷转移已通过TD-DFT方法进行检测。该化合物与 (A) 1TKZ、(B) 4WKE、(C) 5KK7、(D) 5OSC、(E) 6B6G、(F) 6HSN 和 (G) 6YW0 蛋白质受体对接,以找到最佳的配体-蛋白质相互作用,获得的最佳和最低结合能为-5.4 Kcal/mol。还对标题分子及其不同衍生物进行了药物相似性分析,以确认标题分子的药物样特征。报告形成键的原子的杂化。标题分子的电荷转移已通过TD-DFT方法进行检测。该化合物与 (A) 1TKZ、(B) 4WKE、(C) 5KK7、(D) 5OSC、(E) 6B6G、(F) 6HSN 和 (G) 6YW0 蛋白质受体对接,以找到最佳的配体-蛋白质相互作用,获得的最佳和最低结合能为-5.4 Kcal/mol。还对标题分子及其不同衍生物进行了药物相似性分析,以确认标题分子的药物样特征。报告形成键的原子的杂化。标题分子的电荷转移已通过TD-DFT方法进行检测。该化合物与 (A) 1TKZ、(B) 4WKE、(C) 5KK7、(D) 5OSC、(E) 6B6G、(F) 6HSN 和 (G) 6YW0 蛋白质受体对接,以找到最佳的配体-蛋白质相互作用,获得的最佳和最低结合能为-5.4 Kcal/mol。还对标题分子及其不同衍生物进行了药物相似性分析,以确认标题分子的药物样特征。

































 京公网安备 11010802027423号
京公网安备 11010802027423号