Chemical Physics Letters ( IF 2.8 ) Pub Date : 2021-09-16 , DOI: 10.1016/j.cplett.2021.139055 Yan Yu 1 , Xindi Huang 2 , Hang Yin 3 , Yu Feng 1 , Hua Xuan 1 , Haixiang He 1, 4
|
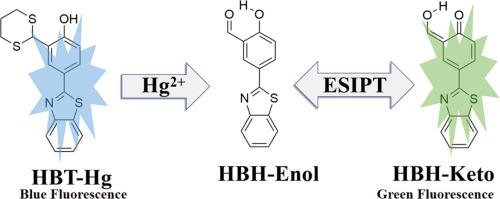
We have investigated the fluorescent mechanism of 4-(benzo[d]thiazol-2-yl)-2-(1,3-dithian-2-yl) phenol (HBT-Hg) and the excited state intramolecular proton transfer processes (ESIPT) for hydroxyphenyl benzothiazole-based fluorescent probe (HBH) by DFT/TDDFT method. After optimizing the geometries of HBH-Enol and HBH-Keto in the ground (S0) and first excited (S1) states at B3LYP/TZVP/IEFPCM computation level, we demonstrated that the intramolecular hydrogen bond was strengthened in S1 state and would facilitate the ESIPT process of HBH, which were further verified by the electron spectroscopy and the frontier molecular orbital results. In addition, the spectral results also show that the isomerism form (HBH-Keto) plays the role in the fluorescence emission. The potential energy curve analyses according to variational O-H coordinate also support proton transfer process because of the ultra-low potential energy barriers for HBH in S1 state. Thus, the intramolecular proton transfer is more likely to occur in the excited state and it turns out to be a spontaneous process.
中文翻译:

苯并噻唑衍生物荧光探针的激发态分子内质子转移机制:自发 ESIPT 过程
我们研究了 4-(benzo[d]thiazol-2-yl)-2-(1,3-dithian-2-yl) phenol (HBT-Hg) 和激发态分子内质子转移过程 (ESIPT) 的荧光机制) 通过 DFT/TDDFT 方法用于基于羟苯基苯并噻唑的荧光探针 (HBH)。在 B3LYP/TZVP/IEFPCM 计算水平优化了 HBH-Enol 和 HBH-Keto 在地面(S 0)和第一激发(S 1)态的几何结构后,我们证明了分子内氢键在 S 1 中得到了加强状态并将促进 HBH 的 ESIPT 过程,这通过电子光谱和前沿分子轨道结果进一步验证。此外,光谱结果还表明异构体形式(HBH-Keto)在荧光发射中起作用。由于处于 S 1态的HBH 具有超低的势能势垒,因此根据变分 OH 坐标分析的势能曲线也支持质子转移过程。因此,分子内质子转移更有可能发生在激发态,并且是自发过程。






























 京公网安备 11010802027423号
京公网安备 11010802027423号