当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Permissive Medium Chain Acyl-CoA Carboxylase Enables the Efficient Biosynthesis of Extender Units for Engineering Polyketide Carbon Scaffolds
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-09-16 , DOI: 10.1021/acscatal.1c03818 Jun Zhang 1, 2 , Mengmeng Zheng 1, 2 , Jiayan Yan 1 , Zixin Deng 1 , Dongqing Zhu 1 , Xudong Qu 1, 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-09-16 , DOI: 10.1021/acscatal.1c03818 Jun Zhang 1, 2 , Mengmeng Zheng 1, 2 , Jiayan Yan 1 , Zixin Deng 1 , Dongqing Zhu 1 , Xudong Qu 1, 2
Affiliation
|
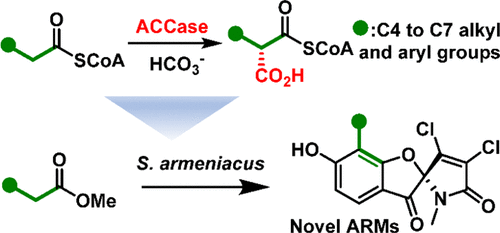
The selective incorporation of new-to-nature extender units into polyketide synthesis is a highly effective way to engineer their carbon scaffolds. Currently, most atypical extender units are biosynthesized via reductive carboxylation of α,β-unsaturated thioesters catalyzed by crotonyl-CoA reductase/carboxylases or thioesterification of malonates by malonyl-CoA ligases followed by epimerase catalyzed enantiomerization. In this study, we identified an unusual β-subunit (Arm13) of the acyl-CoA carboxylase (ACCase) from the biosynthesis of armeniaspirols. This β-subunit is permissive to the α- and ε-subunits of propionyl-CoA carboxylase to form a fully active ACCase. Distinct from the other regular ACCases in substrate specificity, this ACCase can directly carboxylate medium chain acyl-CoAs ranging from C6 to C9 either with or without terminal substituents, e.g., alkyne or phenyl groups, to produce corresponding alkylmalonyl-CoAs with high catalytic efficiency. By harnessing the power of this ACCase in extender unit biosynthesis, we introduced structural variation into the carbon scaffold of armeniaspirols by feeding corresponding carboxylate precursors. These findings not only enrich the knowledge of the medium chain-specific ACCases but also provide an important biocatalyst for diversifying building blocks, which will greatly facilitate polyketide engineering.
中文翻译:

一种允许的中链酰基辅酶 A 羧化酶能够有效地生物合成用于工程聚酮化合物碳支架的扩展单元
在聚酮化合物合成中选择性地掺入新的天然增量剂单元是设计碳支架的一种高效方法。目前,大多数非典型的增量剂单元是通过巴豆酰辅酶A还原酶/羧化酶催化的α,β-不饱和硫酯的还原羧化或丙二酸辅酶A连接酶催化的丙二酸酯的硫酯化,然后是差向异构酶催化的对映异构化来生物合成的。在这项研究中,我们从亚美尼亚螺类的生物合成中鉴定了一种不寻常的酰基辅酶 A 羧化酶 (ACCase) 的 β-亚基 (Arm13)。该β-亚基允许丙酰辅酶A羧化酶的α-和ε-亚基形成完全活性的ACCase。在底物特异性方面与其他常规 ACCase 不同,这种ACCase 可以直接羧化C6 到C9 的中链酰基辅酶A,带有或不带有末端取代基,例如炔或苯基,以高催化效率产生相应的烷基丙二酰辅酶A。通过利用这种 ACCase 在扩展单元生物合成中的力量,我们通过供给相应的羧酸盐前体,将结构变异引入了亚美尼亚螺的碳支架中。这些发现不仅丰富了中链特异性 ACCases 的知识,而且为构建模块的多样化提供了重要的生物催化剂,这将极大地促进聚酮化合物工程。我们通过加入相应的羧酸盐前体,将结构变异引入亚美尼亚螺的碳支架中。这些发现不仅丰富了中链特异性 ACCases 的知识,而且为构建模块的多样化提供了重要的生物催化剂,这将极大地促进聚酮化合物工程。我们通过加入相应的羧酸盐前体,将结构变异引入亚美尼亚螺的碳支架中。这些发现不仅丰富了中链特异性 ACCases 的知识,而且为构建模块的多样化提供了重要的生物催化剂,这将极大地促进聚酮化合物工程。
更新日期:2021-10-01
中文翻译:

一种允许的中链酰基辅酶 A 羧化酶能够有效地生物合成用于工程聚酮化合物碳支架的扩展单元
在聚酮化合物合成中选择性地掺入新的天然增量剂单元是设计碳支架的一种高效方法。目前,大多数非典型的增量剂单元是通过巴豆酰辅酶A还原酶/羧化酶催化的α,β-不饱和硫酯的还原羧化或丙二酸辅酶A连接酶催化的丙二酸酯的硫酯化,然后是差向异构酶催化的对映异构化来生物合成的。在这项研究中,我们从亚美尼亚螺类的生物合成中鉴定了一种不寻常的酰基辅酶 A 羧化酶 (ACCase) 的 β-亚基 (Arm13)。该β-亚基允许丙酰辅酶A羧化酶的α-和ε-亚基形成完全活性的ACCase。在底物特异性方面与其他常规 ACCase 不同,这种ACCase 可以直接羧化C6 到C9 的中链酰基辅酶A,带有或不带有末端取代基,例如炔或苯基,以高催化效率产生相应的烷基丙二酰辅酶A。通过利用这种 ACCase 在扩展单元生物合成中的力量,我们通过供给相应的羧酸盐前体,将结构变异引入了亚美尼亚螺的碳支架中。这些发现不仅丰富了中链特异性 ACCases 的知识,而且为构建模块的多样化提供了重要的生物催化剂,这将极大地促进聚酮化合物工程。我们通过加入相应的羧酸盐前体,将结构变异引入亚美尼亚螺的碳支架中。这些发现不仅丰富了中链特异性 ACCases 的知识,而且为构建模块的多样化提供了重要的生物催化剂,这将极大地促进聚酮化合物工程。我们通过加入相应的羧酸盐前体,将结构变异引入亚美尼亚螺的碳支架中。这些发现不仅丰富了中链特异性 ACCases 的知识,而且为构建模块的多样化提供了重要的生物催化剂,这将极大地促进聚酮化合物工程。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号