当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dual-Site Cooperation for High Benzyl Alcohol Oxidation Activity of MnO2 in Biphasic MnOx–CeO2 Catalyst Using Aerial O2 in the Vapor Phase
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2021-09-16 , DOI: 10.1021/acs.jpcc.1c04158 Tanmoy Mazumder 1, 2 , Sudip Dandapat 1, 2 , Tinku Baidya 1 , Pravin R. Likhar 1, 2 , Adam H. Clark 3 , Parthasarathi Bera 4 , Khushubo Tiwari 5 , Soumitra Payra 6 , Bolla Srinivasa Rao 1 , Sounak Roy 6 , Krishanu Biswas 5
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2021-09-16 , DOI: 10.1021/acs.jpcc.1c04158 Tanmoy Mazumder 1, 2 , Sudip Dandapat 1, 2 , Tinku Baidya 1 , Pravin R. Likhar 1, 2 , Adam H. Clark 3 , Parthasarathi Bera 4 , Khushubo Tiwari 5 , Soumitra Payra 6 , Bolla Srinivasa Rao 1 , Sounak Roy 6 , Krishanu Biswas 5
Affiliation
|
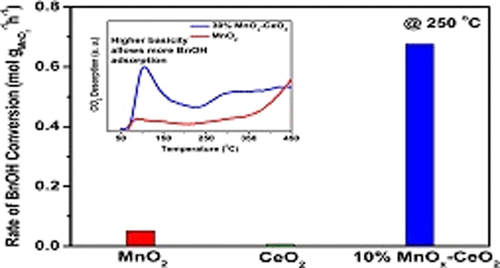
Selective oxidation of benzyl alcohol to benzaldehyde in the vapor phase has drawn growing interest recently. In this work, MnOx–CeO2 mixed oxide compositions have been prepared by a coprecipitation method and tested for their oxidation activities of benzyl alcohol to benzaldehyde in the vapor phase. Detailed structural analyses have indicated that MnOx–CeO2 catalysts contain two phases, namely, α-MnO2 and fluorite CeO2 phases. The benzyl alcohol oxidation activity of pure MnO2 is more than 7 times higher compared to that of CeO2, indicating a much higher intrinsic oxidation ability of the MnO2 phase. Further, enhancement of the benzyl alcohol oxidation rate over MnO2 in 10%MnOx–CeO2 catalyst by 13 times is observed in relation to pure MnO2. The role of CeO2 in the MnOx–CeO2 catalyst has also been investigated, which indicates that the oxidation activity is almost independent of CeO2. However, stronger adsorption of benzyl alcohol over the MnOx–CeO2 catalysts compared to that of MnO2 points to the role of CeO2 in adsorption. Thus, both the CeO2 and the MnO2 components have different roles in the catalytic process—adsorption of benzyl alcohol on the CeO2 surface and its oxidation on MnO2 at the interface. The cooperation between the two sites toward oxidation could happen due to jumping of adsorbed benzyl alcohol from the surface of the CeO2 phase to the closest Mn4+ site in the MnO2 phase at the contact surface with MnO2 by thermal motion.
中文翻译:

在气相中使用空气中的 O2 在双相 MnOx-CeO2 催化剂中实现 MnO2 高苄醇氧化活性的双位点合作
苯甲醇在气相中选择性氧化为苯甲醛最近引起了越来越多的兴趣。在这项工作中,MnO x -CeO 2混合氧化物组合物已通过共沉淀法制备,并测试了它们在气相中将苯甲醇氧化成苯甲醛的活性。详细的结构分析表明,MnO x -CeO 2催化剂包含两相,即α-MnO 2和萤石CeO 2相。纯MnO 2的苯甲醇氧化活性比CeO 2高7倍以上,表明MnO 2具有更高的内在氧化能力阶段。此外,观察到与纯 MnO 2 相比,在 10% MnO x -CeO 2催化剂中苯甲醇氧化速率比 MnO 2提高了13 倍。CeO 2在MnO x -CeO 2催化剂中的作用也已被研究,这表明氧化活性几乎与CeO 2无关。然而,苄醇在所述的MnO吸附更强X -CeO 2个催化剂相比的MnO 2点的CeO的作用2中吸附。因此,CeO 2和 MnO 2组分在催化过程中具有不同的作用——苯甲醇在 CeO 2表面的吸附和在界面处在MnO 2上的氧化。由于热运动,吸附的苯甲醇从CeO 2相的表面跃迁到MnO 2相中与MnO 2接触表面最近的Mn 4+位点,从而可能发生两个位点之间的氧化合作。
更新日期:2021-09-30
中文翻译:

在气相中使用空气中的 O2 在双相 MnOx-CeO2 催化剂中实现 MnO2 高苄醇氧化活性的双位点合作
苯甲醇在气相中选择性氧化为苯甲醛最近引起了越来越多的兴趣。在这项工作中,MnO x -CeO 2混合氧化物组合物已通过共沉淀法制备,并测试了它们在气相中将苯甲醇氧化成苯甲醛的活性。详细的结构分析表明,MnO x -CeO 2催化剂包含两相,即α-MnO 2和萤石CeO 2相。纯MnO 2的苯甲醇氧化活性比CeO 2高7倍以上,表明MnO 2具有更高的内在氧化能力阶段。此外,观察到与纯 MnO 2 相比,在 10% MnO x -CeO 2催化剂中苯甲醇氧化速率比 MnO 2提高了13 倍。CeO 2在MnO x -CeO 2催化剂中的作用也已被研究,这表明氧化活性几乎与CeO 2无关。然而,苄醇在所述的MnO吸附更强X -CeO 2个催化剂相比的MnO 2点的CeO的作用2中吸附。因此,CeO 2和 MnO 2组分在催化过程中具有不同的作用——苯甲醇在 CeO 2表面的吸附和在界面处在MnO 2上的氧化。由于热运动,吸附的苯甲醇从CeO 2相的表面跃迁到MnO 2相中与MnO 2接触表面最近的Mn 4+位点,从而可能发生两个位点之间的氧化合作。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号