当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Novel Pyrazolo[3,4-d]pyrimidin-4-one Derivatives as Potential Antifungal Agents: Design, Synthesis, and Biological Evaluation
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2021-09-15 , DOI: 10.1021/acs.jafc.1c02454 Xiang Cheng 1 , Wei Wang 1, 2 , Yunxiao Wang 1 , Dongguo Xia 1 , Fang Yin 2 , Qiaoyun Liu 1 , Huisheng Luo 1 , Meng Li 1 , Chengqi Zhang 3 , Haiqun Cao 3 , Xianhai Lv 1, 3
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2021-09-15 , DOI: 10.1021/acs.jafc.1c02454 Xiang Cheng 1 , Wei Wang 1, 2 , Yunxiao Wang 1 , Dongguo Xia 1 , Fang Yin 2 , Qiaoyun Liu 1 , Huisheng Luo 1 , Meng Li 1 , Chengqi Zhang 3 , Haiqun Cao 3 , Xianhai Lv 1, 3
Affiliation
|
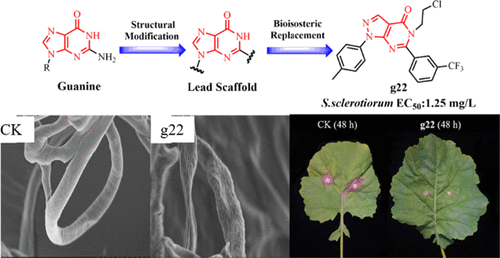
Plant pathogenic fungi seriously threaten agricultural production. There is an urgent need to develop novel fungicides with low toxicity and high efficiency. In this study, we designed and synthesized 44 pyrazolo[3,4-d]pyrimidin-4-one derivatives and evaluated them for their fungicidal activities. The bioassay data revealed that most of the target compounds possessed moderate to high in vitro antifungal activities. Especially compound g22 exhibited remarkable antifungal activity against Sclerotinia sclerotiorum with an EC50 value of 1.25 mg/L, close to that of commercial fungicide boscalid (EC50 = 0.96 mg/L) and fluopyram (EC50 = 1.91 mg/L). Moreover, compound g22 possessed prominent protective activity against S. sclerotiorum in vivo for 24 h (95.23%) and 48 h (93.78%), comparable to positive control boscalid (24 h (96.63%); 48 h (93.23%)). Subsequent studies indicated that compound g22 may impede the growth and reproduction of S. sclerotiorum by affecting the morphology of mycelium, destroying cell membrane integrity, and increasing cell membrane permeability. In addition, the application of compound g22 did not injure the growth or reproduction of Italian bees. This study revealed that compound g22 is expected to be developed for efficient and safe agricultural fungicides.
中文翻译:

新型 Pyrazolo[3,4-d]pyrimidin-4-one 衍生物作为潜在的抗真菌剂:设计、合成和生物学评价
植物病原真菌严重威胁农业生产。迫切需要开发低毒高效的新型杀菌剂。在本研究中,我们设计并合成了 44 种 pyrazolo[3,4- d ]pyrimidin-4-one 衍生物,并评估了它们的杀菌活性。生物测定数据表明,大多数目标化合物具有中到高的体外抗真菌活性。特别是化合物g22对核盘菌表现出显着的抗真菌活性,EC 50值为1.25 mg/L,接近商业杀菌剂啶酰菌胺(EC 50 = 0.96 mg/L)和氟吡菌酰胺(EC 50= 1.91 毫克/升)。此外,化合物g22在体内 24 小时 (95.23%) 和 48 小时 (93.78%)具有显着的抗核盘菌保护活性,与阳性对照啶酰菌胺(24 小时 (96.63%);48 小时 (93.23%))相当。随后的研究表明,化合物g22可能通过影响菌丝体的形态、破坏细胞膜完整性和增加细胞膜通透性来阻碍核盘菌的生长和繁殖。此外,化合物g22的施用并未对意大利蜜蜂的生长或繁殖造成伤害。该研究表明,化合物g22有望开发用于高效安全的农用杀菌剂。
更新日期:2021-09-29
中文翻译:

新型 Pyrazolo[3,4-d]pyrimidin-4-one 衍生物作为潜在的抗真菌剂:设计、合成和生物学评价
植物病原真菌严重威胁农业生产。迫切需要开发低毒高效的新型杀菌剂。在本研究中,我们设计并合成了 44 种 pyrazolo[3,4- d ]pyrimidin-4-one 衍生物,并评估了它们的杀菌活性。生物测定数据表明,大多数目标化合物具有中到高的体外抗真菌活性。特别是化合物g22对核盘菌表现出显着的抗真菌活性,EC 50值为1.25 mg/L,接近商业杀菌剂啶酰菌胺(EC 50 = 0.96 mg/L)和氟吡菌酰胺(EC 50= 1.91 毫克/升)。此外,化合物g22在体内 24 小时 (95.23%) 和 48 小时 (93.78%)具有显着的抗核盘菌保护活性,与阳性对照啶酰菌胺(24 小时 (96.63%);48 小时 (93.23%))相当。随后的研究表明,化合物g22可能通过影响菌丝体的形态、破坏细胞膜完整性和增加细胞膜通透性来阻碍核盘菌的生长和繁殖。此外,化合物g22的施用并未对意大利蜜蜂的生长或繁殖造成伤害。该研究表明,化合物g22有望开发用于高效安全的农用杀菌剂。






























 京公网安备 11010802027423号
京公网安备 11010802027423号