当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Toward Unraveling the Origin of Lithium Fluoride in the Solid Electrolyte Interphase
Chemistry of Materials ( IF 7.2 ) Pub Date : 2021-09-14 , DOI: 10.1021/acs.chemmater.1c01744 Chuntian Cao 1, 2, 3, 4 , Travis P. Pollard 5, 6 , Oleg Borodin 5, 6 , Julian E. Mars 1, 2, 3, 4 , Yuchi Tsao 7 , Maria R. Lukatskaya 1, 4, 8 , Robert M. Kasse 1, 9 , Marshall A. Schroeder 5, 6 , Kang Xu 5, 6 , Michael F. Toney 1, 2, 3, 4 , Hans-Georg Steinrück 1, 4, 10
Chemistry of Materials ( IF 7.2 ) Pub Date : 2021-09-14 , DOI: 10.1021/acs.chemmater.1c01744 Chuntian Cao 1, 2, 3, 4 , Travis P. Pollard 5, 6 , Oleg Borodin 5, 6 , Julian E. Mars 1, 2, 3, 4 , Yuchi Tsao 7 , Maria R. Lukatskaya 1, 4, 8 , Robert M. Kasse 1, 9 , Marshall A. Schroeder 5, 6 , Kang Xu 5, 6 , Michael F. Toney 1, 2, 3, 4 , Hans-Georg Steinrück 1, 4, 10
Affiliation
|
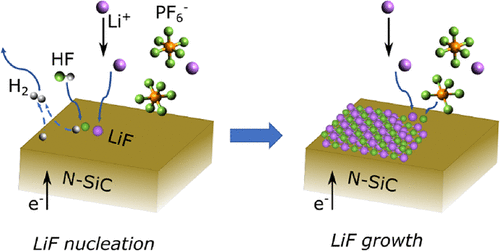
The solid electrolyte interphase (SEI) is an integral part of Li-ion batteries and their performance, representing the key enabler for reversibility and also serving as a major source of capacity loss and dictating the cell kinetics. In the pervasive LiPF6-containing electrolytes, LiF is one of the SEI’s major components; however, its formation mechanism remains unclear. Electrochemically, two separate reduction pathways could lead to LiF, either via direct anion reduction or electrocatalytic transformation of HF. This work aims to shed light on understanding the role played by these pathways. In a multimodal experimental and theoretical approach, we carried out operando structural characterization on an inert model single crystalline N-doped SiC working electrode during voltammetric scans in LiPF6 baseline electrolytes and complemented these with ex situ chemical characterization. These results were supplemented by cyclic voltammetry measurements using a variety of electrolyte formulations under different cycling rates as well as quantum chemical calculations and Born–Oppenheimer molecular dynamics simulations. Our results reveal that the reductive formation of LiF in these systems is likely a combined mechanism, which concomitantly involves both direct anion reduction and electrocatalytic transformation of HF. Specifically, LiF nucleates via the electrocatalytic transformation of HF followed by significant anion reduction.
中文翻译:

揭示固体电解质中间相中氟化锂的起源
固体电解质界面 (SEI) 是锂离子电池及其性能的组成部分,是可逆性的关键推动因素,也是容量损失和决定电池动力学的主要来源。在普遍存在的含LiPF 6的电解质中,LiF 是 SEI 的主要成分之一;但其形成机制尚不清楚。在电化学上,两个独立的还原途径可以通过直接阴离子还原或 HF 的电催化转化产生 LiF。这项工作旨在阐明理解这些途径所起的作用。在多模态实验和理论方法中,我们进行了操作在 LiPF 6基线电解质中进行伏安扫描期间,惰性模型单晶 N 掺杂 SiC 工作电极的结构表征,并通过非原位化学表征对这些进行了补充。这些结果得到了在不同循环速率下使用各种电解质配方的循环伏安测量以及量子化学计算和 Born-Oppenheimer 分子动力学模拟的补充。我们的结果表明,这些系统中 LiF 的还原形成可能是一种组合机制,同时涉及 HF 的直接阴离子还原和电催化转化。具体而言,LiF 通过 HF 的电催化转化随后显着的阴离子还原成核。
更新日期:2021-09-28
中文翻译:

揭示固体电解质中间相中氟化锂的起源
固体电解质界面 (SEI) 是锂离子电池及其性能的组成部分,是可逆性的关键推动因素,也是容量损失和决定电池动力学的主要来源。在普遍存在的含LiPF 6的电解质中,LiF 是 SEI 的主要成分之一;但其形成机制尚不清楚。在电化学上,两个独立的还原途径可以通过直接阴离子还原或 HF 的电催化转化产生 LiF。这项工作旨在阐明理解这些途径所起的作用。在多模态实验和理论方法中,我们进行了操作在 LiPF 6基线电解质中进行伏安扫描期间,惰性模型单晶 N 掺杂 SiC 工作电极的结构表征,并通过非原位化学表征对这些进行了补充。这些结果得到了在不同循环速率下使用各种电解质配方的循环伏安测量以及量子化学计算和 Born-Oppenheimer 分子动力学模拟的补充。我们的结果表明,这些系统中 LiF 的还原形成可能是一种组合机制,同时涉及 HF 的直接阴离子还原和电催化转化。具体而言,LiF 通过 HF 的电催化转化随后显着的阴离子还原成核。

































 京公网安备 11010802027423号
京公网安备 11010802027423号