当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
First-Principles Study of NO Reduction by CO on Cu2O(110) and Pd1/Cu2O(110) Surfaces
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2021-09-13 , DOI: 10.1021/acs.jpcc.1c05806
Hong Wen 1 , Jing-yao Liu 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2021-09-13 , DOI: 10.1021/acs.jpcc.1c05806
Hong Wen 1 , Jing-yao Liu 1
Affiliation
|
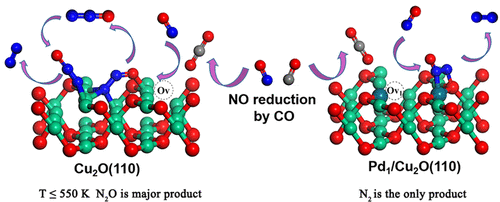
Selective catalytic reduction of NO by CO (CO-SCR) is considered as one of the most effective methods for simultaneous removal of two pollutants. The main challenge to achieve this goal is to develop a low-cost, highly effective, and stable catalyst. In this work, on the basis of experimental study, we designed a Pd1/Cu2O(110) single-atom catalyst to improve the selectivity of Cu2O to N2. The reaction mechanisms of NO reduction by CO on the undoped Cu2O(110) and Pd1/Cu2O(110) were studied by using density functional theory and microkinetic models, and the catalytic performance of the two catalysts was compared. The results showed that both surfaces have high CO oxidation activity. Pd doping improves the adsorption strength of NO and CO and changes the preferential configuration of NO on the surface with oxygen vacancies. Possible reaction pathways for the formation of N2 and N2O were located. Microkinetic analysis showed that the overall NO conversion rate and CO2 formation rate on Pd1/Cu2O(110) are much higher than those on Cu2O(110). Compared with the 100% selectivity of N2O on Cu2O(110) at 300–450 K, doping a single Pd atom into the top layer of Cu2O(110) can obtain 100% N2 selectivity in the whole temperature of 300–1000 K. It is further confirmed that the reaction proceeds via the different mechanism on the undoped and Pd-doped surfaces. N2O is formed on Cu2O(110) via the intermediate NNO, while N2 is formed on Pd1/Cu2O(110) via the dimer ONNO. This study is expected to provide a clue for the design of oxide-supported single-atom catalysts for NO reduction.
中文翻译:

CO在Cu2O(110)和Pd1/Cu2O(110)表面还原NO的第一性原理研究
CO选择性催化还原NO(CO-SCR)被认为是同时去除两种污染物的最有效方法之一。实现这一目标的主要挑战是开发一种低成本、高效且稳定的催化剂。在这项工作中,我们在实验研究的基础上,设计了一种 Pd 1 /Cu 2 O(110) 单原子催化剂,以提高 Cu 2 O 对 N 2的选择性。CO在未掺杂Cu 2 O(110)和Pd 1 /Cu 2上还原NO的反应机理利用密度泛函理论和微动力学模型对O(110)进行了研究,并比较了两种催化剂的催化性能。结果表明,两个表面都具有较高的CO氧化活性。Pd掺杂提高了NO和CO的吸附强度,并改变了表面具有氧空位的NO的优先构型。确定了形成 N 2和 N 2 O 的可能反应途径。微动力学分析表明,Pd 1 /Cu 2 O(110)上的总体NO转化率和CO 2形成率远高于Cu 2 O(110)。与 N 2 O 对 Cu 2的 100% 选择性相比O(110) 在 300-450 K,在 Cu 2 O(110)的顶层掺杂单个 Pd 原子可以获得 100% N 2选择性,在 300-1000 K 的整个温度下。进一步证实了反应通过未掺杂和 Pd 掺杂表面上的不同机制进行。N 2 O通过中间体 NNO在 Cu 2 O(110) 上形成,而 N 2通过二聚体 ONNO在 Pd 1 /Cu 2 O(110) 上形成。该研究有望为氧化物负载单原子催化剂的设计提供线索。
更新日期:2021-09-23
中文翻译:

CO在Cu2O(110)和Pd1/Cu2O(110)表面还原NO的第一性原理研究
CO选择性催化还原NO(CO-SCR)被认为是同时去除两种污染物的最有效方法之一。实现这一目标的主要挑战是开发一种低成本、高效且稳定的催化剂。在这项工作中,我们在实验研究的基础上,设计了一种 Pd 1 /Cu 2 O(110) 单原子催化剂,以提高 Cu 2 O 对 N 2的选择性。CO在未掺杂Cu 2 O(110)和Pd 1 /Cu 2上还原NO的反应机理利用密度泛函理论和微动力学模型对O(110)进行了研究,并比较了两种催化剂的催化性能。结果表明,两个表面都具有较高的CO氧化活性。Pd掺杂提高了NO和CO的吸附强度,并改变了表面具有氧空位的NO的优先构型。确定了形成 N 2和 N 2 O 的可能反应途径。微动力学分析表明,Pd 1 /Cu 2 O(110)上的总体NO转化率和CO 2形成率远高于Cu 2 O(110)。与 N 2 O 对 Cu 2的 100% 选择性相比O(110) 在 300-450 K,在 Cu 2 O(110)的顶层掺杂单个 Pd 原子可以获得 100% N 2选择性,在 300-1000 K 的整个温度下。进一步证实了反应通过未掺杂和 Pd 掺杂表面上的不同机制进行。N 2 O通过中间体 NNO在 Cu 2 O(110) 上形成,而 N 2通过二聚体 ONNO在 Pd 1 /Cu 2 O(110) 上形成。该研究有望为氧化物负载单原子催化剂的设计提供线索。

































 京公网安备 11010802027423号
京公网安备 11010802027423号