当前位置:
X-MOL 学术
›
Appl. Catal. A Gen.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effective and selective oxidation of 2-butanol over Mn supported catalyst systems
Applied Catalysis A: General ( IF 4.7 ) Pub Date : 2016-08-30 06:26:38
Kandasamy Prabu, Marimuthu Prabu, Ashok Kumar Venugopal, Aswathy Thareparambil Venugopalan, W.V.Y. Sai Sandilya, Chinnakonda S. Gopinath, Thirumalaiswamy Raja
Applied Catalysis A: General ( IF 4.7 ) Pub Date : 2016-08-30 06:26:38
Kandasamy Prabu, Marimuthu Prabu, Ashok Kumar Venugopal, Aswathy Thareparambil Venugopalan, W.V.Y. Sai Sandilya, Chinnakonda S. Gopinath, Thirumalaiswamy Raja
|
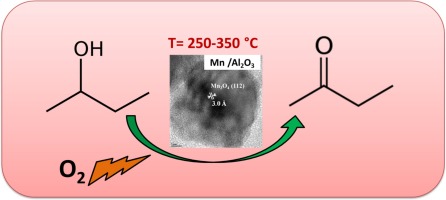
Oxidation of alcohols to their corresponding aldehydes/ketones is an important reaction in industries as well as in academic perspective. Selective oxidation (Selox) of alcohols like methanol, ethanol and propanol are well studied in literature; however, alcohols like butanol, pentanol, octanol is a challenging task. Selective oxidation of 2-butanol to methyl ethyl ketone (MEK) is an important reaction due to its wide range of applications. Herein, we demonstrated the selective oxidation of 2-butanol to MEK over Mn supported on different oxide supports. A series of MnxOy-Al2O3 (MA), MnxOy-CeO2 (MC), MnxOy-ZrO2 (MZ) and MnxOy-SiO2 (MS) catalysts were prepared by co-precipitation followed by hydrothermal method. As synthesised catalysts were characterised by various physico-chemical characterisation techniques. It was found that the presence of Mn3O4 species in MA and MZ catalysts is responsible for maximum catalytic activity towards 2-butanol oxidation. MA catalyst conferred a maximum 2-butanol conversion of 51% and 88% selectivity towards MEK. XPS analysis revealed that Mn in MA catalyst exists in +2 and +3 oxidation states and responsible for 2-butanol oxidation. Moreover it was found that the acidity of the catalyst also plays an important role in catalytic activity.
中文翻译:

在锰负载的催化剂体系上对2-丁醇进行有效和选择性的氧化
醇氧化为相应的醛/酮是工业和学术界的重要反应。文献对醇类(如甲醇,乙醇和丙醇)的选择性氧化(Selox)进行了很好的研究。然而,丁醇,戊醇,辛醇等醇类是一项艰巨的任务。2-丁醇选择性氧化为甲乙酮(MEK)是重要的反应,因为它具有广泛的应用范围。在本文中,我们证明了在不同氧化物载体上的Mn上,2-丁醇选择性氧化为MEK。一系列Mn x O y -Al 2 O 3(MA),Mn x O y -CeO 2(MC),Mn x O y共沉淀然后水热法制得-ZrO 2(MZ)和Mn x O y -SiO 2(MS)催化剂。作为合成的催化剂,通过各种物理化学表征技术进行了表征。发现在MA和MZ催化剂中Mn 3 O 4种类的存在是导致对2-丁醇氧化的最大催化活性的原因。MA催化剂赋予2-丁醇对MEK的最大选择性为51%和88%的选择性。XPS分析表明,MA催化剂中的Mn以+2和+3氧化态存在,并负责2-丁醇的氧化。此外,发现催化剂的酸度在催化活性中也起重要作用。
更新日期:2016-08-31
中文翻译:

在锰负载的催化剂体系上对2-丁醇进行有效和选择性的氧化
醇氧化为相应的醛/酮是工业和学术界的重要反应。文献对醇类(如甲醇,乙醇和丙醇)的选择性氧化(Selox)进行了很好的研究。然而,丁醇,戊醇,辛醇等醇类是一项艰巨的任务。2-丁醇选择性氧化为甲乙酮(MEK)是重要的反应,因为它具有广泛的应用范围。在本文中,我们证明了在不同氧化物载体上的Mn上,2-丁醇选择性氧化为MEK。一系列Mn x O y -Al 2 O 3(MA),Mn x O y -CeO 2(MC),Mn x O y共沉淀然后水热法制得-ZrO 2(MZ)和Mn x O y -SiO 2(MS)催化剂。作为合成的催化剂,通过各种物理化学表征技术进行了表征。发现在MA和MZ催化剂中Mn 3 O 4种类的存在是导致对2-丁醇氧化的最大催化活性的原因。MA催化剂赋予2-丁醇对MEK的最大选择性为51%和88%的选择性。XPS分析表明,MA催化剂中的Mn以+2和+3氧化态存在,并负责2-丁醇的氧化。此外,发现催化剂的酸度在催化活性中也起重要作用。































 京公网安备 11010802027423号
京公网安备 11010802027423号