Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2021-09-11 , DOI: 10.1016/j.bioorg.2021.105352 Ahmed Elkamhawy 1 , Hyeon Jeong Kim 2 , Mohamed H Elsherbeny 3 , Sora Paik 4 , Jong-Hyun Park 5 , Lizaveta Gotina 6 , Magda H Abdellattif 7 , Noha A Gouda 8 , Jungsook Cho 8 , Kyeong Lee 8 , Ae Nim Pae 6 , Ki Duk Park 6 , Eun Joo Roh 9
|
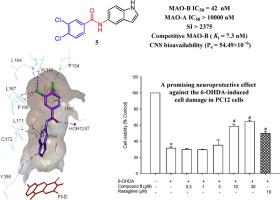
Since there is no disease-modifying treatment discovered yet for Parkinson’s disease (PD), there is still a vital need to develop novel selective monoamine oxidase B (MAO-B) inhibitors as promising therapeutically active candidates for PD patients. Herein, we report the design, synthesis, and full characterization of new twenty-six indole derivatives as potential human MAO-B (hMAO-B) selective inhibitors. Six compounds (2i, 3b–e, and 5) exhibited low micromolar to nanomolar inhibitory activities over hMAO-B; compared to our recently reported N-substituted indole-based lead compound VIII (hMAO-B IC50 = 777 nM), compound 5 (3,4-dichloro-N-(1H-indol-5-yl)benzamide) exhibited 18-fold increase in potency (IC50 = 42 nM). A selectivity study over hMAO-A revealed an excellent selectivity index of compound 5 (SI > 2375) with a 47-fold increase compared to rasagiline (II, a well-known MAO-B inhibitor, SI > 50). A further kinetic evaluation of compound 5 over hMAO-B showed a reversible and competitive mode of inhibition with Ki value of 7 nM. Highly effective permeability and high CNS bioavailability of compound 5 with Pe = 54.49 × 10−6 cm/s were demonstrated. Compound 5 also exhibited a low cytotoxicity profile and a promising neuroprotective effect against the 6-hydroxydopamine-induced neuronal cell damage in PC12 cells, which was more effective than that of rasagiline. Docking simulations on both hMAO-B and hMAO-A supported the in vitro data and served as further molecular evidence. Accordingly, we report the discovery of compound 5 as one of the most potent indole-based MAO-B inhibitors to date which is noteworthy to be further evaluated as a promising agent for PD treatment.
中文翻译:

3,4-二氯-N-(1H-indol-5-yl)benzamide 的发现:一种高效、选择性和竞争性的 hMAO-B 抑制剂,具有高 BBB 通透性和神经保护作用
由于尚未发现帕金森病 (PD) 的疾病改善疗法,因此仍然迫切需要开发新型选择性单胺氧化酶 B (MAO-B) 抑制剂作为 PD 患者的有希望的治疗活性候选物。在此,我们报告了作为潜在的人类 MAO-B ( h MAO-B) 选择性抑制剂的新 26 种吲哚衍生物的设计、合成和完整表征。六种化合物(2i、3b - e和5)对h MAO-B表现出低微摩尔至纳摩尔的抑制活性;与我们最近报道的基于N-取代吲哚的先导化合物VIII ( h MAO-B IC50 = 777 nM),化合物5 (3,4-二氯-N -(1 H-吲哚-5-基)苯甲酰胺)表现出 18 倍的效力增加 (IC 50 = 42 nM)。对h MAO- A 的选择性研究揭示了化合物5的出色选择性指数(SI > 2375),与雷沙吉兰(II,一种众所周知的 MAO-B 抑制剂,SI > 50)相比增加了 47 倍。化合物5对h MAO-B的进一步动力学评估显示了一种可逆的竞争性抑制模式,K i值为 7 nM。化合物5与P的高效渗透性和高CNS生物利用度证明了e = 54.49 × 10 -6 cm/s。化合物5还表现出低细胞毒性特征和对 6-羟基多巴胺诱导的 PC12 细胞神经元细胞损伤的有希望的神经保护作用,这比雷沙吉兰更有效。h MAO-B 和h MAO-A 的对接模拟支持体外数据并作为进一步的分子证据。因此,我们报告发现化合物5是迄今为止最有效的基于吲哚的 MAO-B 抑制剂之一,值得进一步评估作为治疗 PD 的有前途的药物。































 京公网安备 11010802027423号
京公网安备 11010802027423号