当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nickel-Catalyzed Highly Regio- and Stereoselective Cross-Trimerization between Triisopropylsilylacetylene and Internal Alkynes Leading to 1,3-Diene-5-ynes
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2009-03-11 , DOI: 10.1021/ja900146u Kenichi Ogata 1 , Hiroyuki Murayama 1 , Jun Sugasawa 1 , Noriyuki Suzuki 1 , Shin-ichi Fukuzawa 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2009-03-11 , DOI: 10.1021/ja900146u Kenichi Ogata 1 , Hiroyuki Murayama 1 , Jun Sugasawa 1 , Noriyuki Suzuki 1 , Shin-ichi Fukuzawa 1
Affiliation
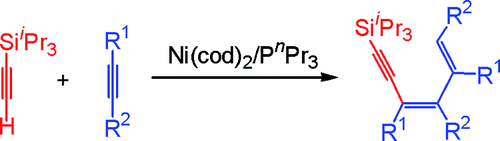
|
The first highly selective 1:2 cross-trimerization between triisopropylsilylacetylene and 2 equiv of internal alkynes, leading to 1,3-diene-5-yne compounds, was achieved using the Ni(cod)(2)/P(n)Pr(3) catalyst. Various symmetrical and asymmetrical internal alkynes could be used for the cross-trimerization reaction with high regio- and stereoselectivity.
中文翻译:

镍催化的三异丙基甲硅烷基乙炔和内炔之间的高度区域和立体选择性交叉三聚反应导致 1,3-二烯-5-炔
使用 Ni(cod)(2)/P(n)Pr() 实现了三异丙基甲硅烷基乙炔和 2 当量的内部炔烃之间的第一个高选择性 1:2 交叉三聚反应,产生了 1,3-二烯-5-炔化合物。 3)催化剂。各种对称和不对称的内炔可用于具有高区域和立体选择性的交叉三聚反应。
更新日期:2009-03-11
中文翻译:

镍催化的三异丙基甲硅烷基乙炔和内炔之间的高度区域和立体选择性交叉三聚反应导致 1,3-二烯-5-炔
使用 Ni(cod)(2)/P(n)Pr() 实现了三异丙基甲硅烷基乙炔和 2 当量的内部炔烃之间的第一个高选择性 1:2 交叉三聚反应,产生了 1,3-二烯-5-炔化合物。 3)催化剂。各种对称和不对称的内炔可用于具有高区域和立体选择性的交叉三聚反应。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号