Medicinal Chemistry ( IF 1.9 ) Pub Date : 2021-09-30 , DOI: 10.2174/1573406416666200826102051 Asma Mukhtar 1 , Shazia Shah 1 , Kanwal 1 , Shehryar Hameed 1 , Khalid Mohammed Khan 1 , Shahid Ullah Khan 2 , Sumera Zaib 2 , Jamshed Iqbal 2 , Shahnaz Parveen 3
|
Background: Diabetes mellitus is one of the most chronic metabolic disorders. Since past few years, our research group had synthesized and evaluated libraries of heterocyclic compounds against α and β-glucosidase enzymes and found encouraging results. The current study comprises of evaluation of indane-1,3-dione as antidiabetic agents based on our previously reported results obtained from closely related moiety isatin and its derivatives.
Objective: A library of twenty three indane-1,3-dione derivatives (1-23) was synthesized and evaluated for α and β-glucosidase inhibitions. Moreover, in silico docking studies were carried out to investigate the putative binding mode of selected compounds with the target enzyme.
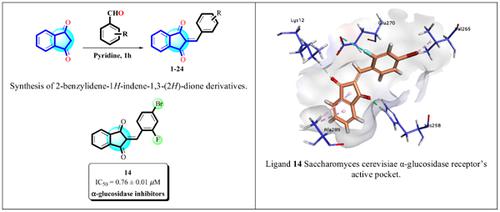
Methods: The indane-1,3-dione derivatives (1-23) were synthesized by Knoevenagel condensation of different substituted benzaldehydes with indane-1,3-dione under basic condition. The structures of synthetic molecules were deduced by using different spectroscopic techniques, including 1H-, 13C-NMR, EI-MS, and CHN analysis. Compounds (1-23) were evaluated for α and β-glucosidase inhibitions by adopting the literature protocols.
Result: Off twenty three, eleven compounds displayed good to moderate activity against α- glucosidase enzyme, nonetheless, all compounds exhibited less than 50% inhibition against β- glucosidase enzyme. Compounds 1, 14, and 23 displayed good activity against α-glucosidase enzyme with IC50 values of 2.80 ± 0.11, 0.76 ± 0.01, and 2.17 ± 0.18 μM, respectively. The results have shown that these compounds have selectively inhibited the α-glucosidase enzyme. The in silico docking studies also supported the above results and showed different types of interactions of synthetic molecules with the active site of enzyme.
Conclusion: The compounds 1, 14, and 23 have shown good inhibition against α-glucosidase and may potentially serve as lead for the development of new therapeutic representatives.
中文翻译:

Indane-1,3-diones:作为潜在和选择性 α-葡萄糖苷酶抑制剂,它们的合成,体外和计算机研究
背景:糖尿病是最慢性的代谢性疾病之一。过去几年,我们的研究小组已经合成和评估了针对α和β-葡萄糖苷酶的杂环化合物库,并发现了令人鼓舞的结果。当前的研究包括基于我们之前报道的从密切相关的靛红部分及其衍生物中获得的结果,评估茚满-1,3-二酮作为抗糖尿病药物。
目的:合成了 23 种茚满-1,3-二酮衍生物 (1-23) 的文库,并评估其对 α 和 β-葡萄糖苷酶的抑制作用。此外,还进行了计算机对接研究,以研究选定化合物与目标酶的推定结合模式。
方法:在碱性条件下,通过不同取代苯甲醛与茚满1,3-二酮的Knoevenagel缩合反应合成茚满1,3-二酮衍生物(1-23)。合成分子的结构是通过使用不同的光谱技术推导出来的,包括1 H-、13 C-NMR、EI-MS 和 CHN 分析。通过采用文献方案评价化合物(1-23)的α和β-葡萄糖苷酶抑制作用。
结果:在 23 种化合物中,11 种化合物对 α-葡萄糖苷酶表现出良好至中等的活性,然而,所有化合物对 β-葡萄糖苷酶的抑制作用均低于 50%。化合物 1、14 和 23 对 α-葡萄糖苷酶显示出良好的活性,IC 50值分别为 2.80 ± 0.11、0.76 ± 0.01 和 2.17 ± 0.18 μM。结果表明,这些化合物选择性地抑制了α-葡萄糖苷酶。计算机对接研究也支持了上述结果,并显示了合成分子与酶活性位点的不同类型的相互作用。
结论:化合物 1、14 和 23 对 α-葡萄糖苷酶显示出良好的抑制作用,并可能成为开发新治疗代表的先导物。































 京公网安备 11010802027423号
京公网安备 11010802027423号