Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
“Water-in-Sugar” Electrolytes Enable Ultrafast and Stable Electrochemical Naked Proton Storage
Small ( IF 13.0 ) Pub Date : 2021-09-09 , DOI: 10.1002/smll.202102375
Zhen Su 1 , Junbo Chen 1 , Wenhao Ren 1 , Haocheng Guo 1 , Chen Jia 1 , Songyan Yin 2 , Junming Ho 1 , Chuan Zhao 1
Small ( IF 13.0 ) Pub Date : 2021-09-09 , DOI: 10.1002/smll.202102375
Zhen Su 1 , Junbo Chen 1 , Wenhao Ren 1 , Haocheng Guo 1 , Chen Jia 1 , Songyan Yin 2 , Junming Ho 1 , Chuan Zhao 1
Affiliation
|
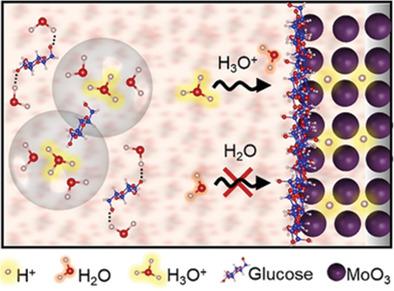
Proton is an ideal charge carrier for rechargeable batteries due to its small ionic radius, ultrafast diffusion kinetics and wide availability. However, in commonly used acid electrolytes, the co-interaction of polarized water and proton (namely hydronium) with electrode materials often causes electrode structural distortions. The hydronium adsorption on electrode surfaces also facilitates hydrogen evolution as an unwanted side reaction. Here, a “water-in-sugar” electrolyte with high concentration of glucose dissolved in acid to enable the naked proton intercalation, as well as an extended 3.9 V working potential window, is shown. A glucose-derived organic thin film is formed on electrode surface upon cycling. Molecular dynamics simulations reveal the significant decrease of free water in bulk electrolytes, while density functional theory calculations indicate that glucose preferentially binds to the electrode surface which can inhibit water adsorption. The scarcity of free water and the protective organic film work in synergy to suppress water interactions with the electrode surface, which enables the naked proton (de)intercalation. The “water-in-sugar” electrolyte significantly enhances a MoO3 electrode for stable cycling over 100 000 times. This facile electrolyte approach opens new avenues to aqueous electrochemistry and energy storage devices.
中文翻译:

“糖包水”电解质实现超快和稳定的电化学裸质子存储
质子是可充电电池的理想电荷载体,因为它具有较小的离子半径、超快的扩散动力学和广泛的可用性。然而,在常用的酸性电解质中,极化的水和质子(即水合氢)与电极材料的共同相互作用往往会导致电极结构扭曲。电极表面上的水合氢吸附也促进了作为不需要的副反应的析氢。在这里,显示了一种“糖包水”电解质,其中高浓度葡萄糖溶解在酸中,以实现裸质子嵌入,以及扩展的 3.9 V 工作电位窗口。循环时在电极表面形成葡萄糖衍生的有机薄膜。分子动力学模拟揭示了大量电解质中自由水的显着减少,而密度泛函理论计算表明,葡萄糖优先结合到电极表面,可以抑制水的吸附。游离水的稀缺和保护性有机膜协同作用以抑制水与电极表面的相互作用,从而实现裸质子(脱)嵌入。“糖包水”电解质显着增强了 MoO3电极可稳定循环超过 100 000 次。这种简便的电解质方法为水性电化学和储能设备开辟了新的途径。
更新日期:2021-10-06
中文翻译:

“糖包水”电解质实现超快和稳定的电化学裸质子存储
质子是可充电电池的理想电荷载体,因为它具有较小的离子半径、超快的扩散动力学和广泛的可用性。然而,在常用的酸性电解质中,极化的水和质子(即水合氢)与电极材料的共同相互作用往往会导致电极结构扭曲。电极表面上的水合氢吸附也促进了作为不需要的副反应的析氢。在这里,显示了一种“糖包水”电解质,其中高浓度葡萄糖溶解在酸中,以实现裸质子嵌入,以及扩展的 3.9 V 工作电位窗口。循环时在电极表面形成葡萄糖衍生的有机薄膜。分子动力学模拟揭示了大量电解质中自由水的显着减少,而密度泛函理论计算表明,葡萄糖优先结合到电极表面,可以抑制水的吸附。游离水的稀缺和保护性有机膜协同作用以抑制水与电极表面的相互作用,从而实现裸质子(脱)嵌入。“糖包水”电解质显着增强了 MoO3电极可稳定循环超过 100 000 次。这种简便的电解质方法为水性电化学和储能设备开辟了新的途径。

































 京公网安备 11010802027423号
京公网安备 11010802027423号