当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Coordination of the Copper Centers in Particulate Methane Monooxygenase: Comparison between Methanotrophs and Characterization of the CuC Site by EPR and ENDOR Spectroscopies
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-09-09 , DOI: 10.1021/jacs.1c07018 Richard J Jodts 1 , Matthew O Ross 1 , Christopher W Koo 1 , Peter E Doan 1 , Amy C Rosenzweig 1 , Brian M Hoffman 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-09-09 , DOI: 10.1021/jacs.1c07018 Richard J Jodts 1 , Matthew O Ross 1 , Christopher W Koo 1 , Peter E Doan 1 , Amy C Rosenzweig 1 , Brian M Hoffman 1
Affiliation
|
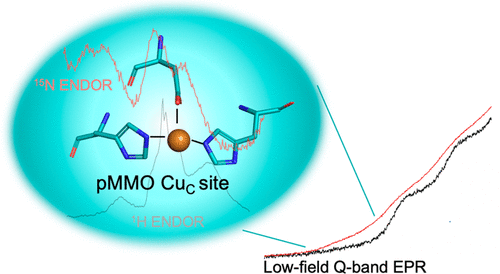
In nature, methane is oxidized to methanol by two enzymes, the iron-dependent soluble methane monooxygenase (sMMO) and the copper-dependent particulate MMO (pMMO). While sMMO’s diiron metal active site is spectroscopically and structurally well-characterized, pMMO’s copper sites are not. Recent EPR and ENDOR studies have established the presence of two monocopper sites, but the coordination environment of only one has been determined, that within the PmoB subunit and denoted CuB. Moreover, this recent work only focused on a type I methanotrophic pMMO, while previous observations of the type II enzyme were interpreted in terms of the presence of a dicopper site. First, this report shows that the type II Methylocystis species strain Rockwell pMMO, like the type I pMMOs, contains two monocopper sites and that its CuB site has a coordination environment identical to that of type I enzymes. As such, for the full range of pMMOs this report completes the refutation of prior and ongoing suggestions of multicopper sites. Second, and of primary importance, EPR/ENDOR measurements (a) for the first time establish the coordination environment of the spectroscopically observed site, provisionally denoted CuC, in both types of pMMO, thereby (b) establishing the assignment of this site observed by EPR to the crystallographically observed metal-binding site in the PmoC subunit. Finally, these results further indicate that CuC is the likely site of biological methane oxidation by pMMO, a conclusion that will serve as a foundation for proposals regarding the mechanism of this reaction.
中文翻译:

颗粒甲烷单加氧酶中铜中心的协调:甲烷氧化菌与 EPR 和 ENDOR 光谱法表征 CuC 位点的比较
在自然界中,甲烷被两种酶氧化成甲醇,即依赖铁的可溶性甲烷单加氧酶 (sMMO) 和依赖铜的颗粒 MMO (pMMO)。虽然 sMMO 的二铁金属活性位点在光谱和结构上都得到了很好的表征,但 pMMO 的铜位点却不是。最近的 EPR 和 ENDOR 研究已经确定了两个单铜位点的存在,但仅确定了一个的协调环境,即在 PmoB 亚基内并表示为 Cu B。此外,最近的这项工作仅关注 I 型甲烷氧化 pMMO,而先前对 II 型酶的观察被解释为存在二铜位点。首先,本报告显示,II 型甲基孢子虫物种菌株 Rockwell pMMO 与 I 型 pMMO 一样,含有两个单铜位点,其 Cu B位点具有与 I 型酶相同的配位环境。因此,对于所有 pMMO,本报告完成了对先前和正在进行的多铜站点建议的反驳。其次,也是最重要的,EPR/ENDOR 测量 (a) 首次在两种类型的 pMMO 中建立了光谱观测站点的协调环境,临时表示为 Cu C,从而 (b) 确定了观测站点的分配通过 EPR 到 PmoC 亚基中晶体学观察到的金属结合位点。最后,这些结果进一步表明,Cu C是 pMMO 生物甲烷氧化的可能位点,这一结论将作为有关该反应机制的建议的基础。
更新日期:2021-09-22
中文翻译:

颗粒甲烷单加氧酶中铜中心的协调:甲烷氧化菌与 EPR 和 ENDOR 光谱法表征 CuC 位点的比较
在自然界中,甲烷被两种酶氧化成甲醇,即依赖铁的可溶性甲烷单加氧酶 (sMMO) 和依赖铜的颗粒 MMO (pMMO)。虽然 sMMO 的二铁金属活性位点在光谱和结构上都得到了很好的表征,但 pMMO 的铜位点却不是。最近的 EPR 和 ENDOR 研究已经确定了两个单铜位点的存在,但仅确定了一个的协调环境,即在 PmoB 亚基内并表示为 Cu B。此外,最近的这项工作仅关注 I 型甲烷氧化 pMMO,而先前对 II 型酶的观察被解释为存在二铜位点。首先,本报告显示,II 型甲基孢子虫物种菌株 Rockwell pMMO 与 I 型 pMMO 一样,含有两个单铜位点,其 Cu B位点具有与 I 型酶相同的配位环境。因此,对于所有 pMMO,本报告完成了对先前和正在进行的多铜站点建议的反驳。其次,也是最重要的,EPR/ENDOR 测量 (a) 首次在两种类型的 pMMO 中建立了光谱观测站点的协调环境,临时表示为 Cu C,从而 (b) 确定了观测站点的分配通过 EPR 到 PmoC 亚基中晶体学观察到的金属结合位点。最后,这些结果进一步表明,Cu C是 pMMO 生物甲烷氧化的可能位点,这一结论将作为有关该反应机制的建议的基础。































 京公网安备 11010802027423号
京公网安备 11010802027423号