当前位置:
X-MOL 学术
›
Macromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Substituted Thiols in Dynamic Thiol–Thioester Reactions
Macromolecules ( IF 5.1 ) Pub Date : 2021-09-08 , DOI: 10.1021/acs.macromol.1c00649 Nicholas J. Bongiardina 1 , Katelyn F. Long 2 , Maciej Podgórski 3, 4 , Christopher N. Bowman 1, 4
Macromolecules ( IF 5.1 ) Pub Date : 2021-09-08 , DOI: 10.1021/acs.macromol.1c00649 Nicholas J. Bongiardina 1 , Katelyn F. Long 2 , Maciej Podgórski 3, 4 , Christopher N. Bowman 1, 4
Affiliation
|
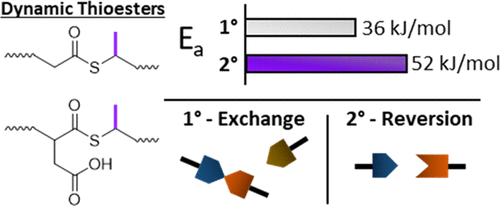
The thiol–thioester reaction has emerged as a promising method for developing covalent adaptable networks (CANs) due to its ability to exchange rapidly under low temperature conditions in a number of solvents, orthogonality among other functional groups, and tunability. Here, the effects of thiol substitution (i.e., primary vs secondary) were assessed with respect to their reactivity in two dynamic thioester reactions: the thiol–thioester exchange and the reversible thiol–anhydride addition. Model NMR experiments were conducted using small-molecule compounds to observe how polymers of similar components would behave in thiol–thioester exchange. It was determined that the Keq was near unity for mixtures of primary thiols and secondary thioesters, and vice versa, in both a polar solvent, DMSO-d6, and at most slightly favors primary thioesters in a relatively nonpolar solvent, CDCl3. Dielectric spectroscopy and stress relaxation experiments were used to determine the relaxation times and activation energies of the two thioester-containing networks: Thiol-ene networks, which undergo thioester exchange, displayed activation energies of 73 and 71 kJ/mol from dielectric measurements and 36 and 53 kJ/mol from stress relaxation for the primary and secondary thiols, respectively. Thiol–anhydride-ene networks, which undergo both thioester exchange and reversible thiol–anhydride addition, displayed activation energies of 94 and 114 kJ/mol from dielectric and 111 and 139 kJ/mol from stress relaxation for primary and secondary thiols, respectively. In both types of networks, the secondary thioester-based networks demonstrated slower dynamics as compared to the same primary network by at least one order of magnitude. In the anhydride network, the secondary thiol also biased the dynamics toward reversible addition.
中文翻译:

动态硫醇-硫酯反应中的取代硫醇
硫醇-硫酯反应已成为开发共价适应性网络 (CAN) 的一种有前途的方法,因为它能够在低温条件下在多种溶剂中快速交换,其他官能团之间的正交性以及可调性。在这里,硫醇取代(即初级与二级)的影响评估了它们在两个动态硫酯反应中的反应性:硫醇 - 硫酯交换和可逆的硫醇 - 酸酐加成。使用小分子化合物进行模型核磁共振实验,以观察相似组分的聚合物在硫醇-硫酯交换中的行为。确定了伯硫醇和仲硫酯的混合物的K eq接近统一,反之亦然,在极性溶剂 DMSO-d 6,并且至多略微有利于相对非极性溶剂CDCl 3 中的伯硫酯. 介电光谱和应力松弛实验用于确定两种含硫酯网络的弛豫时间和活化能:硫醇-烯网络进行硫酯交换,介电测量显示的活化能为 73 和 71 kJ/mol,36 和伯硫醇和仲硫醇的应力松弛分别为 53 kJ/mol。硫醇-酸酐-烯网络经过硫酯交换和可逆硫醇-酸酐加成,显示出来自介电质的 94 和 114 kJ/mol 的活化能,以及来自伯硫醇和仲硫醇的应力松弛活化能分别为 111 和 139 kJ/mol。在这两种类型的网络中,与相同的初级网络相比,基于二级硫酯的网络表现出较慢的动态至少一个数量级。
更新日期:2021-09-28
中文翻译:

动态硫醇-硫酯反应中的取代硫醇
硫醇-硫酯反应已成为开发共价适应性网络 (CAN) 的一种有前途的方法,因为它能够在低温条件下在多种溶剂中快速交换,其他官能团之间的正交性以及可调性。在这里,硫醇取代(即初级与二级)的影响评估了它们在两个动态硫酯反应中的反应性:硫醇 - 硫酯交换和可逆的硫醇 - 酸酐加成。使用小分子化合物进行模型核磁共振实验,以观察相似组分的聚合物在硫醇-硫酯交换中的行为。确定了伯硫醇和仲硫酯的混合物的K eq接近统一,反之亦然,在极性溶剂 DMSO-d 6,并且至多略微有利于相对非极性溶剂CDCl 3 中的伯硫酯. 介电光谱和应力松弛实验用于确定两种含硫酯网络的弛豫时间和活化能:硫醇-烯网络进行硫酯交换,介电测量显示的活化能为 73 和 71 kJ/mol,36 和伯硫醇和仲硫醇的应力松弛分别为 53 kJ/mol。硫醇-酸酐-烯网络经过硫酯交换和可逆硫醇-酸酐加成,显示出来自介电质的 94 和 114 kJ/mol 的活化能,以及来自伯硫醇和仲硫醇的应力松弛活化能分别为 111 和 139 kJ/mol。在这两种类型的网络中,与相同的初级网络相比,基于二级硫酯的网络表现出较慢的动态至少一个数量级。































 京公网安备 11010802027423号
京公网安备 11010802027423号