Chemistry of Heterocyclic Compounds ( IF 1.4 ) Pub Date : 2021-09-08 , DOI: 10.1007/s10593-021-02982-8 Alexander V. Komkov 1 , Mikhail A. Kozlov 1 , Andrey S. Dmitrenok 1 , Nataliya G. Kolotyrkina 1 , Mikhail E. Minyaev 1 , Igor V. Zavarzin 1
|
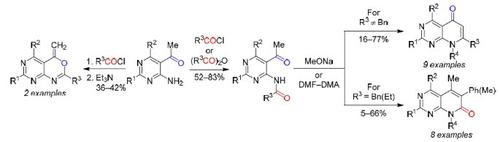
Methods were developed for the synthesis of new pyrido[2,3-d]pyrimidine and pyrimidino[4,5-d][1,3]oxazine derivatives. When heated under reflux with MeONa in BuOH, 5-acetyl-4-aminopyrimidines, acylated with carboxylic anhydrides or acid chlorides, are transformed into pyrido[2,3-d]pyrimidin-5-one derivatives, i.e., the acetyl methyl group and the amide carbonyl moiety are involved in the cyclization. In the presence of an activated CH2 group (e.g., PhCH2) in the amide moiety, the cyclization involves this group and the acetyl carbonyl giving rise to pyrido[2,3-d]pyrimidin-7-one derivatives. The reaction of 5-acetyl-4-aminopyrimidines with carboxylic acid chlorides under reflux in xylene followed by addition of a base affords pyrimidino[4,5-d][1,3]oxazines.
中文翻译:

从 5-合成新的吡啶并[2,3-d]嘧啶-5-one、吡啶并[2,3-d]嘧啶-7-one和嘧啶并[4,5-d][1,3]恶嗪衍生物乙酰-4-氨基嘧啶
开发了合成新的吡啶并[2,3- d ]嘧啶和嘧啶并[4,5- d ][1,3]恶嗪衍生物的方法。当与 MeONa 在 BuOH 中回流加热时,用羧酸酐或酰氯酰化的 5-乙酰基-4-氨基嘧啶转化为吡啶并[2,3 - d ]嘧啶-5-酮衍生物,即乙酰甲基和酰胺羰基部分参与环化。在活化的CH的存在2基团(例如。,物理信道2)在酰胺部分中,环化包括该组和乙酰羰基引起吡啶并[2,3- d]嘧啶-7-one衍生物。5-乙酰基-4-氨基嘧啶与羧酸氯化物在二甲苯中回流反应,然后加入碱,得到嘧啶并[4,5- d ][1,3]恶嗪。















































 京公网安备 11010802027423号
京公网安备 11010802027423号