Chemistry of Heterocyclic Compounds ( IF 1.4 ) Pub Date : 2021-09-07 , DOI: 10.1007/s10593-021-02993-5 Aleksey V. Shastin 1 , Artem O. Petrov 1 , Georgiy V. Malkov 1 , Tatiana N. Gavrishova 1
|
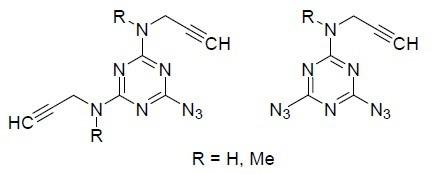
Efficient methods for the synthesis of novel azidopropargylamino-substituted 1,3,5-triazines were developed. 4,6-Diazido-N-(prop-2-yn-1-yl)-1,3,5-triazin-2-amine was obtained by the method of sequential nucleophilic substitution of chlorine atoms in cyanuric chloride by amino and azido groups or by the method of selective substitution of the azido group in triazidotriazine with propargylamine. 6-Azido-N2,N4-dimethyl-N2,N4-di(prop-2-yn-1-yl)-1,3,5-triazine-2,4-diamine was obtained by nitrosation of the corresponding 6-hydrazinyl-N2,N4-dimethyl-N2,N4-di(prop-2-yn-1-yl)-1,3,5-triazine-2,4-diamine. Monomers with a lower melting point were obtained by N-methylation of azidopropargylamino-substituted 1,3,5-triazines.
中文翻译:

叠氮炔丙基氨基取代的 1,3,5-三嗪的合成——用于生产高能聚合物的新型单体
开发了合成新型叠氮炔基氨基取代 1,3,5-三嗪的有效方法。4,6-二叠氮基-N- (prop-2-yn-1-yl)-1,3,5-triazin-2-胺采用氨基和叠氮基依次亲核取代氰尿酰氯中的氯原子的方法得到基团或通过用炔丙胺选择性取代三叠氮基三嗪中的叠氮基的方法。6-叠氮基-N 2 , N 4 -二甲基-N 2 , N 4 -di(prop-2-yn-1-yl) -1,3,5-triazine -2,4-diamine 通过亚硝化得到对应的6-肼基-N 2 , N 4 -二甲基-N 2, N 4 -di(prop-2-yn-1-yl)-1,3,5-triazine-2,4-diamine。具有较低熔点的单体通过叠氮炔炔氨基取代的 1,3,5-三嗪的N-甲基化获得。































 京公网安备 11010802027423号
京公网安备 11010802027423号