当前位置:
X-MOL 学术
›
ACS Synth. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
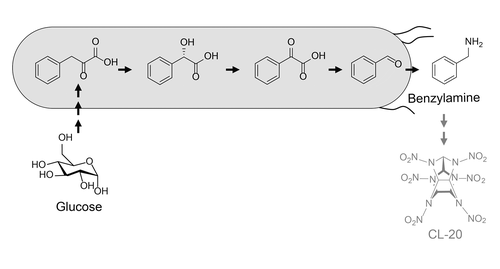
Four-Step Pathway from Phenylpyruvate to Benzylamine, an Intermediate to the High-Energy Propellant CL-20
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2021-09-07 , DOI: 10.1021/acssynbio.1c00021 Ramesh Prasad Pandey 1, 2 , Arturo Casini 2 , Christopher A Voigt 1, 2 , D Benjamin Gordon 1, 2
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2021-09-07 , DOI: 10.1021/acssynbio.1c00021 Ramesh Prasad Pandey 1, 2 , Arturo Casini 2 , Christopher A Voigt 1, 2 , D Benjamin Gordon 1, 2
Affiliation
|
Benzylamine is a commodity chemical used in the synthesis of motion-sickness treatments and anticonvulsants, in dyeing textiles, and as a precursor to the high-energy propellant CL-20. Because chemical production generates toxic waste streams, biosynthetic alternatives have been explored, recently resulting in a functional nine-step pathway from central metabolism (phenylalanine) in E. coli. We report a novel four-step pathway for benzylamine production, which generates the product from cellular phenylpyruvate using enzymes from different sources: a mandelate synthase (Amycolatopsis orientalis), a mandelate oxidase (Streptomyces coelicolor), a benzoylformate decarboxylase (Pseudomonas putida), and an aminotransferase (Salicibacter pomeroyi). This pathway produces benzylamine at 24 mg/L in 15 h (4.5% yield) in cultures of unoptimized cells supplemented with phenylpyruvate. Because the yield is low, supplementation with pathway intermediates is used to troubleshoot the design. This identifies conversion inefficiencies in the mandelate synthase-mediated synthesis of (S)-mandelic acid, and subsequent genome mining identifies a new mandelate synthase (Streptomyces sp. 1114.5) with improved yield. Supplementation experiments also reveal native redirection of ambient phenylpyruvate away from the pathway to phenylalanine. Overall, this work illustrates how retrosynthetic design can dramatically reduce the number of enzymes in a pathway, potentially reducing its draw on cellular resources. However, it also shows that such benefits can be abrogated by inefficiencies of individual conversions. Addressing these barriers can provide an alternative approach to green production of benzylamine, eliminating upstream dependence on chlorination chemistry.
中文翻译:

从苯丙酮酸到苄胺的四步途径,苄胺是高能推进剂 CL-20 的中间体
苄胺是一种商品化学品,用于晕车治疗和抗惊厥药的合成、纺织品染色以及高能推进剂 CL-20 的前体。由于化学生产会产生有毒废物流,因此已经探索了生物合成替代品,最近从大肠杆菌中的中央代谢(苯丙氨酸)产生了一个功能性的九步途径。我们报告了一种新的苄胺生产四步途径,该途径使用来自不同来源的酶从细胞苯丙酮酸中生成产物:扁桃酸合酶(Amycolatopsis orientalis)、扁桃酸氧化酶(天蓝色链霉菌)、苯甲酰甲酸脱羧酶(恶臭假单胞菌)和转氨酶(Salicibacter pomeroyi )。在补充有苯丙酮酸的未优化细胞培养物中,该途径在 15 小时内产生 24 mg/L 的苄胺(4.5% 产率)。由于产率低,补充途径中间体用于解决设计问题。这确定了扁桃酸合酶介导的 ( S )-扁桃酸合成中的转化效率低下,随后的基因组挖掘确定了一种新的扁桃酸合酶 ( Streptomyces )sp。1114.5) 提高产量。补充实验还揭示了环境苯丙酮酸的天然重定向远离苯丙氨酸的途径。总体而言,这项工作说明了逆合成设计如何显着减少途径中酶的数量,从而可能减少其对细胞资源的利用。然而,它也表明,这些好处可能会因个人转换效率低下而被取消。解决这些障碍可以提供一种绿色生产苄胺的替代方法,消除上游对氯化化学的依赖。
更新日期:2021-09-17
中文翻译:

从苯丙酮酸到苄胺的四步途径,苄胺是高能推进剂 CL-20 的中间体
苄胺是一种商品化学品,用于晕车治疗和抗惊厥药的合成、纺织品染色以及高能推进剂 CL-20 的前体。由于化学生产会产生有毒废物流,因此已经探索了生物合成替代品,最近从大肠杆菌中的中央代谢(苯丙氨酸)产生了一个功能性的九步途径。我们报告了一种新的苄胺生产四步途径,该途径使用来自不同来源的酶从细胞苯丙酮酸中生成产物:扁桃酸合酶(Amycolatopsis orientalis)、扁桃酸氧化酶(天蓝色链霉菌)、苯甲酰甲酸脱羧酶(恶臭假单胞菌)和转氨酶(Salicibacter pomeroyi )。在补充有苯丙酮酸的未优化细胞培养物中,该途径在 15 小时内产生 24 mg/L 的苄胺(4.5% 产率)。由于产率低,补充途径中间体用于解决设计问题。这确定了扁桃酸合酶介导的 ( S )-扁桃酸合成中的转化效率低下,随后的基因组挖掘确定了一种新的扁桃酸合酶 ( Streptomyces )sp。1114.5) 提高产量。补充实验还揭示了环境苯丙酮酸的天然重定向远离苯丙氨酸的途径。总体而言,这项工作说明了逆合成设计如何显着减少途径中酶的数量,从而可能减少其对细胞资源的利用。然而,它也表明,这些好处可能会因个人转换效率低下而被取消。解决这些障碍可以提供一种绿色生产苄胺的替代方法,消除上游对氯化化学的依赖。






























 京公网安备 11010802027423号
京公网安备 11010802027423号