当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dual-Multivalent-Aptamer-Conjugated Nanoprobes for Superefficient Discerning of Single Circulating Tumor Cells in a Microfluidic Chip with Inductively Coupled Plasma Mass Spectrometry Detection
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2021-09-02 , DOI: 10.1021/acsami.1c11953 Xuan Zhang 1 , Xing Wei 1 , Xue Men 1 , Cheng-Xin Wu 1 , Jun-Jie Bai 1 , Wei-Tao Li 1 , Ting Yang 1 , Ming-Li Chen 1 , Jian-Hua Wang 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2021-09-02 , DOI: 10.1021/acsami.1c11953 Xuan Zhang 1 , Xing Wei 1 , Xue Men 1 , Cheng-Xin Wu 1 , Jun-Jie Bai 1 , Wei-Tao Li 1 , Ting Yang 1 , Ming-Li Chen 1 , Jian-Hua Wang 1
Affiliation
|
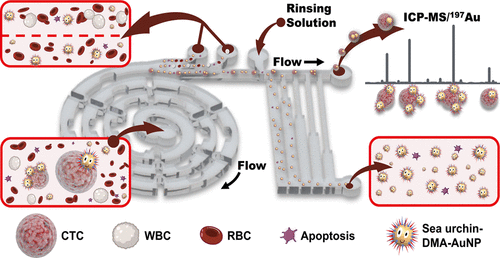
The efficient recognition of circulating tumor cells (CTCs) with an aptamer probe confers numerous benefits; however, the stability and binding affinity of aptamers are significantly hampered in real biological sample matrices. Inspired by the efficient preying mechanism by multiplex tubing feet and endoskeletons of sea urchins, we engineered a superefficient biomimetic single-CTC recognition platform by conjugating dual-multivalent-aptamers (DMAs) Sgc8 and SYL3C onto AuNPs to form a sea urchin-like nanoprobe (sea urchin-DMA-AuNPs). Aptamers Sgc8 and SYL3C selectively bind with the biomarker proteins PTK7 and EpCAM expressed on the surface of CTCs. CTCs were captured with 100% efficiency, followed by sorting on a specially designed multifunctional microfluidic configuration, integrating a single-CTC separation unit and a hydrodynamic filtrating purification unit. After sorting, background-free analysis of biomarker proteins in single CTCs was undertaken with inductively coupled plasma mass spectrometry by measuring the amount of 197Au isotope in sea urchin-DMA-AuNPs. With respect to a single-aptamer nanoprobe/-interface, the dual-aptamer nanoprobe improves the binding efficiency by more than 200% (Kd < 0.35 nM). The microchip facilitates the recognition of single CTCs with a sorting separation rate of 93.6% at a flow rate of 60 μL min–1, and it exhibits 73.8 ± 5.0% measurement efficiency for single CTCs. The present strategy ensures the manipulation and detection of a single CTC in 100 μL of whole blood within 1 h.
中文翻译:

双多价适配体共轭纳米探针用于超高效识别微流控芯片中的单个循环肿瘤细胞,采用电感耦合等离子体质谱检测
使用适体探针有效识别循环肿瘤细胞 (CTC) 带来了许多好处;然而,适体的稳定性和结合亲和力在实际生物样品基质中受到显着阻碍。受多重管脚和海胆内骨骼的高效捕食机制的启发,我们通过将双多价适体 (DMA) Sgc8 和 SYL3C 缀合到 AuNPs 上形成类似海胆的纳米探针,设计了一个超高效的仿生单 CTC 识别平台。海胆-DMA-AuNPs)。适体 Sgc8 和 SYL3C 选择性地与 CTC 表面表达的生物标志物蛋白 PTK7 和 EpCAM 结合。以 100% 的效率捕获 CTC,然后在专门设计的多功能微流体配置上进行分类,集成了一个单一的CTC分离单元和一个流体动力过滤净化单元。分选后,使用电感耦合等离子体质谱法对单个 CTC 中的生物标志物蛋白进行无背景分析,方法是测量海胆中的197 Au 同位素-DMA-AuNPs。对于单适配体纳米探针/界面,双适配体纳米探针的结合效率提高了200%以上(K d < 0.35 nM)。该微芯片有助于识别单个 CTC,在 60 μL min -1的流速下分选分离率为 93.6%,对单个 CTC 的测量效率为 73.8 ± 5.0%。本策略确保在 1 小时内对 100 μL 全血中的单个 CTC 进行操作和检测。
更新日期:2021-09-15
中文翻译:

双多价适配体共轭纳米探针用于超高效识别微流控芯片中的单个循环肿瘤细胞,采用电感耦合等离子体质谱检测
使用适体探针有效识别循环肿瘤细胞 (CTC) 带来了许多好处;然而,适体的稳定性和结合亲和力在实际生物样品基质中受到显着阻碍。受多重管脚和海胆内骨骼的高效捕食机制的启发,我们通过将双多价适体 (DMA) Sgc8 和 SYL3C 缀合到 AuNPs 上形成类似海胆的纳米探针,设计了一个超高效的仿生单 CTC 识别平台。海胆-DMA-AuNPs)。适体 Sgc8 和 SYL3C 选择性地与 CTC 表面表达的生物标志物蛋白 PTK7 和 EpCAM 结合。以 100% 的效率捕获 CTC,然后在专门设计的多功能微流体配置上进行分类,集成了一个单一的CTC分离单元和一个流体动力过滤净化单元。分选后,使用电感耦合等离子体质谱法对单个 CTC 中的生物标志物蛋白进行无背景分析,方法是测量海胆中的197 Au 同位素-DMA-AuNPs。对于单适配体纳米探针/界面,双适配体纳米探针的结合效率提高了200%以上(K d < 0.35 nM)。该微芯片有助于识别单个 CTC,在 60 μL min -1的流速下分选分离率为 93.6%,对单个 CTC 的测量效率为 73.8 ± 5.0%。本策略确保在 1 小时内对 100 μL 全血中的单个 CTC 进行操作和检测。































 京公网安备 11010802027423号
京公网安备 11010802027423号