当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Comprehensive Insight into the Mechanisms of Dopamine in Disrupting Aβ Protofibrils and Inhibiting Aβ Aggregation
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2021-09-02 , DOI: 10.1021/acschemneuro.1c00306 Yujie Chen 1 , Xuhua Li 2 , Chendi Zhan 1 , Zenghui Lao 1 , Fangying Li 1 , Xuewei Dong 1 , Guanghong Wei 1
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2021-09-02 , DOI: 10.1021/acschemneuro.1c00306 Yujie Chen 1 , Xuhua Li 2 , Chendi Zhan 1 , Zenghui Lao 1 , Fangying Li 1 , Xuewei Dong 1 , Guanghong Wei 1
Affiliation
|
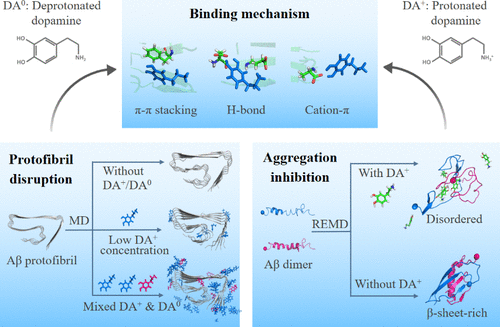
Fibrillary aggregates of amyloid-β (Aβ) are the pathological hallmark of Alzheimer’s disease (AD). Clearing Aβ deposition or inhibiting Aβ aggregation is a promising approach to treat AD. Experimental studies reported that dopamine (DA), an important neurotransmitter, can inhibit Aβ aggregation and disrupt Aβ fibrils in a dose-dependent manner. However, the underlying molecular mechanisms still remain mostly elusive. Herein, we investigated the effect of DA on Aβ42 protofibrils at three different DA-to-Aβ molar ratios (1:1, 2:1, and 10:1) using all-atom molecular dynamics simulations. Our simulations demonstrate that protonated DA at a DA-to-Aβ ratio of 2:1 exhibits stronger Aβ protofibril disruptive capacity than that at a molar-ratio of 1:1 by mostly disrupting the F4-L34-V36 hydrophobic core. When the ratio of DA-to-Aβ increases to 10:1, DA has a high probability to bind to the outer surface of protofibril and has negligible effect on the protofibril structure. Interestingly, at the same DA-to-Aβ ratio (10:1), a mixture of protonated (DA+) and deprotonated (DA0) DA molecules significantly disrupts Aβ protofibrils by the binding of DA0 to the F4-L34-V36 hydrophobic core. Replica-exchange molecular dynamics simulations of Aβ42 dimer show that DA+ inhibits the formation of β-sheets, K28-A42/K28-D23 salt-bridges, and interpeptide hydrophobic interactions and results in disordered coil-rich Aβ dimers, which would inhibit the subsequent fibrillization of Aβ. Further analyses reveal that DA disrupts Aβ protofibril and prevents Aβ dimerization mostly through π–π stacking interactions with residues F4, H6, and H13, hydrogen bonding interactions with negatively charged residues D7, E11, E22 and D23, and cation−π interactions with residues R5. This study provides a complete picture of the molecular mechanisms of DA in disrupting Aβ protofibril and inhibiting Aβ aggregation, which could be helpful for the design of potent drug candidates for the treatment/intervention of AD.
中文翻译:

全面了解多巴胺破坏 Aβ 原纤维和抑制 Aβ 聚集的机制
淀粉样蛋白-β (Aβ) 的纤维聚集体是阿尔茨海默病 (AD) 的病理标志。清除 Aβ 沉积或抑制 Aβ 聚集是治疗 AD 的有希望的方法。实验研究报告说,多巴胺(DA)是一种重要的神经递质,可以以剂量依赖性方式抑制 Aβ 聚集并破坏 Aβ 原纤维。然而,潜在的分子机制仍然难以捉摸。在此,我们研究了 DA 对 Aβ 42的影响使用全原子分子动力学模拟以三种不同的 DA 与 Aβ 摩尔比(1:1、2:1 和 10:1)的原纤维。我们的模拟表明,通过主要破坏 F4-L34-V36 疏水核心,质子化 DA 在 2:1 的 DA 与 Aβ 比率下表现出比在 1:1 摩尔比下更强的 Aβ 原纤维破坏能力。当 DA 与 Aβ 的比例增加到 10:1 时,DA 很有可能与原纤维的外表面结合,对原纤维结构的影响可以忽略不计。有趣的是,在相同的 DA 与 Aβ 比率 (10:1) 下,质子化 (DA + ) 和去质子化 (DA 0 ) DA 分子的混合物通过 DA 0的结合显着破坏 Aβ 原纤维到 F4-L34-V36 疏水核心。Aβ 42二聚体的复制交换分子动力学模拟表明 DA +抑制 β 折叠、K28-A42/K28-D23 盐桥和肽间疏水相互作用的形成,并导致无序的富含螺旋的 Aβ 二聚体,这将抑制随后的 Aβ 原纤维化。进一步的分析表明,DA 主要通过与残基 F4、H6 和 H13 的 π-π 堆积相互作用、与带负电荷的残基 D7、E11、E22 和 D23 的氢键相互作用以及阳离子-π 与残基的相互作用来破坏 Aβ 原纤维并阻止 Aβ 二聚化R5。本研究提供了 DA 破坏 Aβ 原纤维和抑制 Aβ 聚集的分子机制的完整图景,这可能有助于设计用于治疗/干预 AD 的有效候选药物。
更新日期:2021-11-03
中文翻译:

全面了解多巴胺破坏 Aβ 原纤维和抑制 Aβ 聚集的机制
淀粉样蛋白-β (Aβ) 的纤维聚集体是阿尔茨海默病 (AD) 的病理标志。清除 Aβ 沉积或抑制 Aβ 聚集是治疗 AD 的有希望的方法。实验研究报告说,多巴胺(DA)是一种重要的神经递质,可以以剂量依赖性方式抑制 Aβ 聚集并破坏 Aβ 原纤维。然而,潜在的分子机制仍然难以捉摸。在此,我们研究了 DA 对 Aβ 42的影响使用全原子分子动力学模拟以三种不同的 DA 与 Aβ 摩尔比(1:1、2:1 和 10:1)的原纤维。我们的模拟表明,通过主要破坏 F4-L34-V36 疏水核心,质子化 DA 在 2:1 的 DA 与 Aβ 比率下表现出比在 1:1 摩尔比下更强的 Aβ 原纤维破坏能力。当 DA 与 Aβ 的比例增加到 10:1 时,DA 很有可能与原纤维的外表面结合,对原纤维结构的影响可以忽略不计。有趣的是,在相同的 DA 与 Aβ 比率 (10:1) 下,质子化 (DA + ) 和去质子化 (DA 0 ) DA 分子的混合物通过 DA 0的结合显着破坏 Aβ 原纤维到 F4-L34-V36 疏水核心。Aβ 42二聚体的复制交换分子动力学模拟表明 DA +抑制 β 折叠、K28-A42/K28-D23 盐桥和肽间疏水相互作用的形成,并导致无序的富含螺旋的 Aβ 二聚体,这将抑制随后的 Aβ 原纤维化。进一步的分析表明,DA 主要通过与残基 F4、H6 和 H13 的 π-π 堆积相互作用、与带负电荷的残基 D7、E11、E22 和 D23 的氢键相互作用以及阳离子-π 与残基的相互作用来破坏 Aβ 原纤维并阻止 Aβ 二聚化R5。本研究提供了 DA 破坏 Aβ 原纤维和抑制 Aβ 聚集的分子机制的完整图景,这可能有助于设计用于治疗/干预 AD 的有效候选药物。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号