当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Spin-States of Diastereomeric Iron(II) Complexes of 2,6-Bis(thiazolin-2-yl)pyridine (ThioPyBox) Ligands and a Comparison with the Corresponding PyBox Derivatives
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2021-09-02 , DOI: 10.1021/acs.inorgchem.1c01988 Namrah Shahid 1 , Kay E Burrows 1 , Mark J Howard 1 , Christopher M Pask 1 , Oscar Cespedes 2 , Patrick C McGowan 1 , Malcolm A Halcrow 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2021-09-02 , DOI: 10.1021/acs.inorgchem.1c01988 Namrah Shahid 1 , Kay E Burrows 1 , Mark J Howard 1 , Christopher M Pask 1 , Oscar Cespedes 2 , Patrick C McGowan 1 , Malcolm A Halcrow 1
Affiliation
|
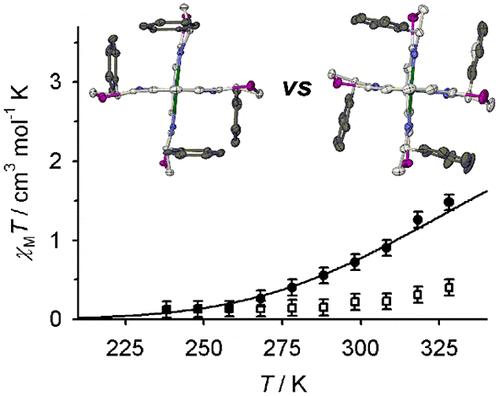
This report investigates homoleptic iron(II) complexes of thiazolinyl analogues of chiral PyBox tridentate ligands: 2,6-bis(4-phenyl-4,5-dihydrothiazol-2-yl)pyridine (L1Ph), 2,6-bis(4-isopropyl-4,5-dihydrothiazol-2-yl)pyridine (L1iPr), and 2,6-bis(4-tert-butyl-4,5-dihydrothiazol-2-yl)pyridine (L1t-Bu). Crystallographic data imply the larger and more flexible thiazolinyl rings reduce steric clashes between the R substituents in homochiral [Fe((R)-L1R)2]2+ or [Fe((S)-L1R)2]2+ (R = Ph, iPr, or t-Bu), compared to their PyBox (L2R) analogues. Conversely, the larger heterocyclic S atoms are in close contact with the R substituents in heterochiral [Fe((R)-L1Ph)((S)-L1Ph)]2+, giving it a more sterically hindered ligand environment than that in [Fe((R)-L2Ph)((S)-L2Ph)]2+ (L2Ph = 2,6-bis(4-phenyl-4,5-dihydrooxazol-2-yl)pyridine). Preformed [Fe((R)-L1Ph)((S)-L1Ph)]2+ and [Fe((R)-L1iPr)((S)-L1iPr)]2+ do not racemize by ligand redistribution in CD3CN solution, but homochiral [Fe(L1iPr)2]2+ and [Fe(L1t-Bu)2]2+ both undergo partial ligand displacement in that solvent. Homochiral [Fe(L1Ph)2]2+ and [Fe(L1iPr)2]2+ exhibit spin-crossover equilibria in CD3CN, centered at 344 ± 6 K and 277 ± 1 K respectively, while their heterochiral congeners are essentially low-spin within the liquid range of the solvent. These data imply that the diastereomers of [Fe(L1Ph)2]2+ and [Fe(L1iPr)2]2+ show a greater difference in their spin-state behaviors than was previous found for [Fe(L2Ph)2]2+. Gas-phase DFT calculations (B86PW91/def2-SVP) of the [Fe(L1R)2]2+ and [Fe(L2R)2]2+ complexes reproduce most of the observed trends, but they overstabilize the high-spin state of SCO-active [Fe(L1iPr)2]2+ by ca. 1.5 kcal mol–1. This might reflect the influence of intramolecular dispersion interactions on the spin states of these compounds. Attempts to model this with the dispersion-corrected functionals B97-D2 or PBE-D3 were less successful than our original protocol, confirming that the spin states of sterically hindered molecules are a challenging computational problem.
中文翻译:

2,6-双(噻唑啉-2-基)吡啶 (ThioPyBox) 配体的非对映异构铁 (II) 配合物的自旋状态以及与相应 PyBox 衍生物的比较
本报告研究了手性 PyBox 三齿配体的噻唑啉基类似物的均质铁 (II) 配合物:2,6-双(4-苯基-4,5-二氢噻唑-2-基) 吡啶 ( L 1 Ph), 2,6-双(4-异丙丙基-4,5-二氢噻唑-2-基)吡啶(大号1我PR),和2,6-双(4-叔丁基- 4,5-二氢吡啶-2-基)吡啶(大号1吨-Bu)。晶体学数据意味着更大和更灵活的噻唑啉环减少了同手性 [Fe(( R )- L 1 R) 2 ] 2+ 中R 取代基之间的空间冲突或 [Fe(( S )- L 1 R) 2 ] 2+ (R = Ph、i Pr 或t -Bu),与它们的 PyBox ( L 2 R) 类似物相比。相反,较大的杂环 S 原子与杂手性 [Fe(( R )- L 1 Ph)(( S )- L 1 Ph)] 2+ 中的 R 取代基紧密接触,使其比在 [Fe(( R )- L 2 Ph)(( S )- L 2 Ph)] 2+ (L 2 Ph = 2,6-双(4-苯基-4,5-二氢恶唑-2-基)吡啶)。预制 [Fe(( R )- L 1 Ph)(( S )- L 1 Ph)] 2+和 [Fe(( R )- L 1 i Pr)(( S )- L 1 i Pr)] 2+在 CD 3 CN 溶液中不通过配体重新分布外消旋化,而是同手性 [Fe( L 1 i Pr) 2 ] 2+和 [Fe( L 1 t -Bu) 2 ]2+都在该溶剂中发生部分配体置换。同手性 [Fe( L 1 Ph) 2 ] 2+和 [Fe( L 1 i Pr) 2 ] 2+在 CD 3 CN 中表现出自旋交叉平衡,分别以 344 ± 6 K 和 277 ± 1 K 为中心,而它们的异手性同源物在溶剂的液体范围内基本上是低自旋的。这些数据意味着 [Fe( L 1 Ph) 2 ] 2+和 [Fe( L 1 i Pr) 2 ] 2+的非对映异构体与之前发现的 [Fe( L 2 Ph) 2 ] 2+相比,它们的自旋态行为显示出更大的差异。[Fe( L 1 R) 2 ] 2+和 [Fe( L 2 R) 2 ] 2+配合物的气相 DFT 计算 (B86PW91/def2-SVP)再现了大部分观察到的趋势,但它们过度稳定了高-自旋状态的 SCO 活性 [Fe( L 1 i Pr) 2 ] 2+由约。1.5 大卡摩尔–1. 这可能反映了分子内分散相互作用对这些化合物的自旋状态的影响。尝试使用色散校正函数 B97-D2 或 PBE-D3 对此进行建模的尝试不如我们的原始协议成功,这证实了空间位阻分子的自旋状态是一个具有挑战性的计算问题。
更新日期:2021-09-20
中文翻译:

2,6-双(噻唑啉-2-基)吡啶 (ThioPyBox) 配体的非对映异构铁 (II) 配合物的自旋状态以及与相应 PyBox 衍生物的比较
本报告研究了手性 PyBox 三齿配体的噻唑啉基类似物的均质铁 (II) 配合物:2,6-双(4-苯基-4,5-二氢噻唑-2-基) 吡啶 ( L 1 Ph), 2,6-双(4-异丙丙基-4,5-二氢噻唑-2-基)吡啶(大号1我PR),和2,6-双(4-叔丁基- 4,5-二氢吡啶-2-基)吡啶(大号1吨-Bu)。晶体学数据意味着更大和更灵活的噻唑啉环减少了同手性 [Fe(( R )- L 1 R) 2 ] 2+ 中R 取代基之间的空间冲突或 [Fe(( S )- L 1 R) 2 ] 2+ (R = Ph、i Pr 或t -Bu),与它们的 PyBox ( L 2 R) 类似物相比。相反,较大的杂环 S 原子与杂手性 [Fe(( R )- L 1 Ph)(( S )- L 1 Ph)] 2+ 中的 R 取代基紧密接触,使其比在 [Fe(( R )- L 2 Ph)(( S )- L 2 Ph)] 2+ (L 2 Ph = 2,6-双(4-苯基-4,5-二氢恶唑-2-基)吡啶)。预制 [Fe(( R )- L 1 Ph)(( S )- L 1 Ph)] 2+和 [Fe(( R )- L 1 i Pr)(( S )- L 1 i Pr)] 2+在 CD 3 CN 溶液中不通过配体重新分布外消旋化,而是同手性 [Fe( L 1 i Pr) 2 ] 2+和 [Fe( L 1 t -Bu) 2 ]2+都在该溶剂中发生部分配体置换。同手性 [Fe( L 1 Ph) 2 ] 2+和 [Fe( L 1 i Pr) 2 ] 2+在 CD 3 CN 中表现出自旋交叉平衡,分别以 344 ± 6 K 和 277 ± 1 K 为中心,而它们的异手性同源物在溶剂的液体范围内基本上是低自旋的。这些数据意味着 [Fe( L 1 Ph) 2 ] 2+和 [Fe( L 1 i Pr) 2 ] 2+的非对映异构体与之前发现的 [Fe( L 2 Ph) 2 ] 2+相比,它们的自旋态行为显示出更大的差异。[Fe( L 1 R) 2 ] 2+和 [Fe( L 2 R) 2 ] 2+配合物的气相 DFT 计算 (B86PW91/def2-SVP)再现了大部分观察到的趋势,但它们过度稳定了高-自旋状态的 SCO 活性 [Fe( L 1 i Pr) 2 ] 2+由约。1.5 大卡摩尔–1. 这可能反映了分子内分散相互作用对这些化合物的自旋状态的影响。尝试使用色散校正函数 B97-D2 或 PBE-D3 对此进行建模的尝试不如我们的原始协议成功,这证实了空间位阻分子的自旋状态是一个具有挑战性的计算问题。






























 京公网安备 11010802027423号
京公网安备 11010802027423号