Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
通过向电子供体引入五元杂环增强锌卟啉染料的光电性能:密度泛函理论和瞬态密度泛函理论研究
ACS Omega ( IF 3.7 ) Pub Date : 2021-09-02 , DOI: 10.1021/acsomega.1c03635 Qingtang Yuan 1 , Yanmin Yu 1 , Zhicheng Sun 2 , Xufeng Song 1
Affiliation
|
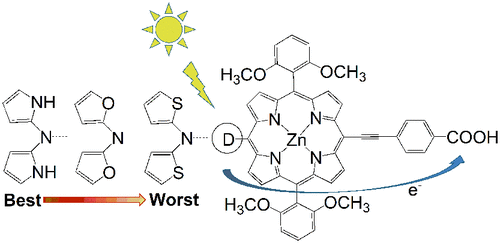
为了制备用于染料敏化太阳能电池的高效染料敏化剂,基于最高效的染料之一YD2- o -C8,通过在电子供体中引入富电子杂环,设计了新型锌卟啉染料敏化剂。通过用吡咯基、呋喃基和噻吩基取代 YD2- o -C8 中供体的苯基,获得了五种潜在有效的染料 Dye1-5。使用密度泛函理论和瞬态密度泛函理论方法研究了所设计染料的电子结构、吸收光谱、分子内电荷转移特性和激发态寿命。所有设计的染料都表现出比YD2- o -C8更好的光电性能。与YD2- o -C8相比,设计的新型染料具有更小的前沿分子轨道能隙和Q波段明显的红移吸收光谱。电荷密度差图和分子内电荷转移特性的分析表明,设计的染料可以更好地促进分子内电荷转移和电子空穴分离。在五种设计的染料中,具有吡咯基的 Dye1 表现出最好的性能。分别具有甲基呋喃基和甲基噻吩基的染料 3 和染料 5 表现出次佳的性能。分别具有呋喃基和噻吩基的染料 2 和染料 4 表现最差。引入的甲基可以进一步提高杂环的给电子能力,促进Q带红移和染料分子内电荷转移。 新型染料的激发态寿命顺序为:YD2- o -C8<Dye4<Dye2<Dye5<Dye3<Dye1,这表明它们向半导体薄膜注入电子的能力更强。

"点击查看英文标题和摘要"

































 京公网安备 11010802027423号
京公网安备 11010802027423号