当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of the C4–C16 Polyketide Fragment of Portimines A and B
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-09-01 , DOI: 10.1021/acs.joc.1c01463
Xiao-Bo Ding 1 , Harry R M Aitken 1 , Esperanza S Pearl 1 , Daniel P Furkert 1, 2 , Margaret A Brimble 1, 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-09-01 , DOI: 10.1021/acs.joc.1c01463
Xiao-Bo Ding 1 , Harry R M Aitken 1 , Esperanza S Pearl 1 , Daniel P Furkert 1, 2 , Margaret A Brimble 1, 2
Affiliation
|
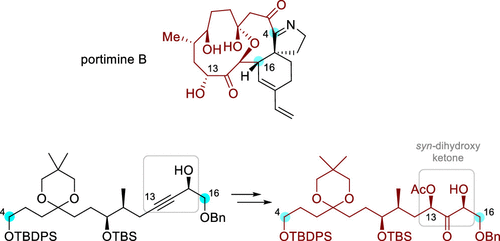
Stereoselective synthesis of the C4–C16 polyketide fragment of portimines A and B is reported, enabled by our previously established method for the stereoselective synthesis of syn-α,α′-dihydroxyketones. The preparation of this advanced fragment provides insights useful for the total synthesis of portimines A and B. An asymmetric Evans aldol reaction was used to install the C10–C11 adjacent stereogenic centers before incorporation of indantrione, followed by epoxidation and epoxide opening to forge the challenging syn-α,α′-dihydroxyketone functionality.
中文翻译:

Portimine A 和 B 的 C4-C16 聚酮化合物片段的合成
报告了portimine A 和B 的C4-C16 聚酮化合物片段的立体选择性合成,通过我们先前建立的syn -α,α'-二羟基酮立体选择性合成方法实现。这一先进片段的制备为总合成 A 和 B 提供了有用的见解。 在掺入阴丹三酮之前,使用不对称埃文斯羟醛反应安装 C10-C11 相邻的立体中心,然后环氧化和环氧化物开口以形成具有挑战性的syn -α,α'-二羟基酮官能团。
更新日期:2021-09-17
中文翻译:

Portimine A 和 B 的 C4-C16 聚酮化合物片段的合成
报告了portimine A 和B 的C4-C16 聚酮化合物片段的立体选择性合成,通过我们先前建立的syn -α,α'-二羟基酮立体选择性合成方法实现。这一先进片段的制备为总合成 A 和 B 提供了有用的见解。 在掺入阴丹三酮之前,使用不对称埃文斯羟醛反应安装 C10-C11 相邻的立体中心,然后环氧化和环氧化物开口以形成具有挑战性的syn -α,α'-二羟基酮官能团。

































 京公网安备 11010802027423号
京公网安备 11010802027423号