当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bisulfite Addition Compounds as Substrates for Reductive Aminations in Water
Organic Letters ( IF 4.9 ) Pub Date : 2021-09-02 , DOI: 10.1021/acs.orglett.1c02604 Xiaohan Li 1 , Karthik S Iyer 1 , Ruchita R Thakore 1 , David K Leahy 2 , J Daniel Bailey 2 , Bruce H Lipshutz 1
Organic Letters ( IF 4.9 ) Pub Date : 2021-09-02 , DOI: 10.1021/acs.orglett.1c02604 Xiaohan Li 1 , Karthik S Iyer 1 , Ruchita R Thakore 1 , David K Leahy 2 , J Daniel Bailey 2 , Bruce H Lipshutz 1
Affiliation
|
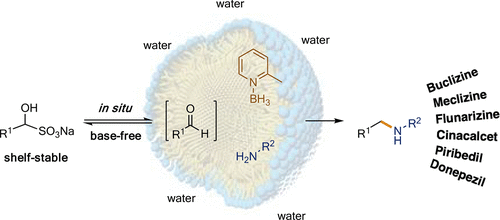
Highly valued products resulting from reductive aminations utilizing shelf-stable bisulfite addition compounds of aldehydes can be made under aqueous micellar catalysis conditions. Readily available α-picolineborane serves as the stoichiometric hydride source. Recycling of the aqueous reaction medium is easily accomplished, and several applications to targets in the pharmaceutical industry are documented.
中文翻译:

亚硫酸氢盐加成化合物作为水中还原胺化的底物
可以在水性胶束催化条件下制备由醛的耐贮存亚硫酸氢盐加成化合物还原胺化产生的高价值产品。容易获得的 α-甲基吡啶硼烷用作化学计量氢化物源。水性反应介质的回收很容易实现,并且记录了在制药工业中对目标的几种应用。
更新日期:2021-09-17
中文翻译:

亚硫酸氢盐加成化合物作为水中还原胺化的底物
可以在水性胶束催化条件下制备由醛的耐贮存亚硫酸氢盐加成化合物还原胺化产生的高价值产品。容易获得的 α-甲基吡啶硼烷用作化学计量氢化物源。水性反应介质的回收很容易实现,并且记录了在制药工业中对目标的几种应用。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号